Page 1003 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1003
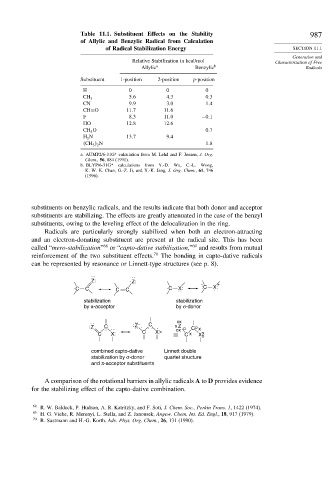
Table 11.1. Substituent Effects on the Stability 987
of Allylic and Benzylic Radical from Calculation
of Radical Stabilization Energy SECTION 11.1
Generation and
Relative Stabilization in kcal/mol Characterization of Free
Allylic a Benzylic b Radicals
Substituent 1-position 2-position p-position
H 0 0 0
5 6 4 3 0 3
CH 3
CN 9 9 3 0 1 4
CH=O 11 7 11 6
F 8 3 11 0 −0 1
HO 12 8 12 6
CH 3 O 0 7
H 2 N 13 7 9 4
CH 3
2 N 1 8
a. AUMP2/6-31G* calculation from M. Lehd and F. Jensen, J. Org.
Chem., 56, 884 (1991).
b. BLYP/6-31G* calculations from Y.-D. Wu, C.-L. Wong,
K. W. K. Chan, G.-Z. Ji, and X.-K. Jang, J. Org. Chem., 61, 746
(1996).
substituents on benzylic radicals, and the results indicate that both donor and acceptor
substituents are stabilizing. The effects are greatly attenuated in the case of the benzyl
substituents, owing to the leveling effect of the delocalization in the ring.
Radicals are particularly strongly stabilized when both an electron-attracting
and an electron-donating substituent are present at the radical site. This has been
69
68
called “mero-stabilization” or “capto-dative stabilization,” and results from mutual
reinforcement of the two substituent effects. 70 The bonding in capto-dative radicals
can be represented by resonance or Linnett-type structures (see p. 8).
: :
Z: . Z:
. C C C C . C : : X – :C . : X +
stabilization stabilization
by π-acceptor by σ-donor
ox
C
:
Z : : C : : Z – . : . . . x o Z x o C o x
.
C X: C X: + C x X o x
combined capto-dative Linnett double
stabilization by σ-donor quartet structure
and π-acceptor substituents
A comparison of the rotational barriers in allylic radicals A to D provides evidence
for the stabilizing effect of the capto-dative combination.
68 R. W. Baldock, P. Hudson, A. R. Katritzky, and F. Soti, J. Chem. Soc., Perkin Trans. 1, 1422 (1974).
69 H. G. Viehe, R. Merenyi, L. Stella, and Z. Janousek, Angew. Chem. Int. Ed. Engl., 18, 917 (1979).
70
R. Sustmann and H.-G. Korth, Adv. Phys. Org. Chem., 26, 131 (1990).