Page 1005 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1005
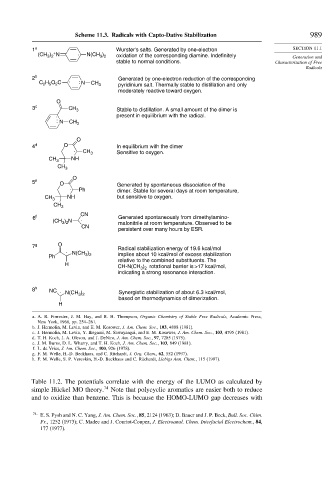
Scheme 11.3. Radicals with Capto-Dative Stabilization 989
1 a Wurster's salts. Generated by one-electron SECTION 11.1
+
(CH ) N . N(CH ) oxidation of the corresponding diamine. Indefinitely Generation and
3 2
3 2
stable to normal conditions. Characterization of Free
Radicals
2 b Generated by one-electron reduction of the corresponding
C H O C . : N CH 3 pyridinium salt. Thermally stable to distillation and only
2
2 5
moderately reactive toward oxygen.
O
3 c CH 3 Stable to distillation. A small amount of the dimer is
. present in equilibrium with the radical.
: N CH 3
O
4 d O In equilibrium with the dimer
. CH 3 Sensitive to oxygen.
CH 3 NH
CH 3
O
5 e O Generated by spontaneous dissociation of the
. Ph dimer. Stable for several days at room temperature,
CH 3 NH but sensitive to oxygen.
CH 3
CN
6 f . Generated spontaneously from dimethylamino-
(CH ) N malonitrile at room temperature. Observed to be
3 2
CN
persistent over many hours by ESR.
7 g O Radical stabilization energy of 19.6 kcal/mol
. N(CH )
Ph 3 2 implies about 10 kcal/mol of excess stabilization
relative to the combined substituents. The
H CH-N(CH ) rotational barrier is >17 kcal/mol,
3 2
indicating a strong resonance interaction.
8 h NC .
)
N(CH 3 2 Synergistic stabilization of about 6.3 kcal/mol,
based on thermodynamics of dimerization.
H
a. A. R. Forrester, J. M. Hay, and R. H. Thompson, Organic Chemistry of Stable Free Radicals, Academic Press,
New York, 1968, pp. 254–261.
b. J. Hermolin, M. Levin, and E. M. Kosower, J. Am. Chem. Soc., 103, 4808 (1981).
c. J. Hermolin, M. Levin, Y. Ikegami, M. Sawayangai, and E. M. Kosower, J. Am. Chem. Soc., 103, 4795 (1981).
d. T. H. Koch, J. A. Oleson, and J. DeNiro, J. Am. Chem. Soc., 97, 7285 (1975).
e. J. M. Burns, D. L. Wharry, and T. H. Koch, J. Am. Chem. Soc., 103, 849 (1981).
f. L. de Vries, J. Am. Chem. Soc., 100, 926 (1978).
g. F. M. Welle, H.-D. Beckhaus, and C. Rüchardt, J. Org. Chem., 62, 552 (1997).
h. F. M. Welle, S. P. Verevkin, H.-D. Beckhaus and C. Rüchardt, Liebigs Ann. Chem., 115 (1997).
Table 11.2. The potentials correlate with the energy of the LUMO as calculated by
simple Hückel MO theory. 74 Note that polycyclic aromatics are easier both to reduce
and to oxidize than benzene. This is because the HOMO-LUMO gap decreases with
74
E. S. Pysh and N. C. Yang, J. Am. Chem. Soc., 85, 2124 (1963); D. Bauer and J. P. Beck, Bull. Soc. Chim.
Fr., 1252 (1973); C. Madec and J. Courtot-Coupez, J. Electroanal. Chem. Interfacial Electrochem., 84,
177 (1977).