Page 1014 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1014
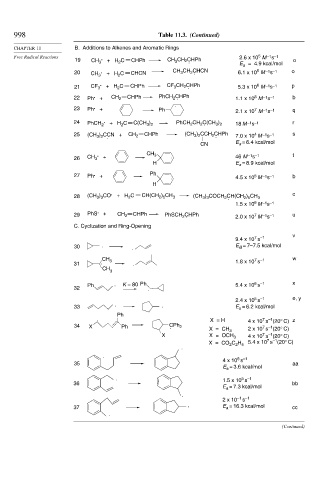
998 Table 11.3. (Continued)
CHAPTER 11 B. Additions to Alkenes and Aromatic Rings
5
–1 –1
Free Radical Reactions . 2.6 x 10 M s
19 CH 3 + H C CHPh CH CH 2 CHPh E = 4.9 kcal/mol o
.
3
2
a
5
–1 –1
2 .
20 . + H C CHCN CH CH CHCN 6.1 x 10 M s o
3
CH 3 2
6
21 CF 3 . + H C CHPh CF 3 CH 2 CHPh 5.3 x 10 M s p
–1 –1
.
2
8
22 Ph . + CH 2 CHPh PhCH CHPh 1.1 x 10 M s b
.
–1 –1
2
.
23 Ph . + Ph 2.1 x 10 M s q
7
–1 –1
24 PhCH 2 . + H C C(CH ) PhCH CH C(CH ) 18 M s r
–1 –1
2 .
3 2
3 2
2
2
) CCH CHPh
25 (CH ) CCN + CH 2 CHPh (CH 3 2 2 . 7.0 x 10 M s s
–1 –1
4
3 2 .
= 6.4 kcal/mol
CN E a
–1 –1
26 CH 3 . + CH 3 . 46 M s t
H E a
= 8.9 kcal/mol
27 Ph . + Ph . 4.5 x 10 M s b
5
–1 –1
H
28 (CH ) CO . + H C CH(CH ) CH 3 (CH ) COCH CH(CH ) CH 3 c
2 5
2 .
2
3 3
2 5
3 3
–1 –1
6
1.5 x 10 M s
29 PhS . + CH 2 CHPh PhSCH CHPh 2.0 x 10 M s u
2 .
–1 –1
7
C. Cyclization and Ring-Opening
v
7 –1
9.4 x 10 s
30 . . E = 7–7.5 kcal/mol
a
CH 3 7 –1 w
31 . . 1.8 x 10 s
CH
3
6 –1
Ph . K = 80 Ph 5.4 x 10 s x
32 .
5 –1
2.4 x 10 s e, y
33 . . E a = 6.2 kcal/mol
Ph
. X = H 4 x 10 s (20° C) z
7 –1
34 X Ph CPh 2 7 –1
.
X = CH 3 2 x 10 s (20° C)
7 –1
X X = OCH 3 4 x 10 s (20° C)
7 –1
X = CO C H 5.4 x 10 s (20° C)
2 2 5
.
.
8 –1
4 x 10 s
35 aa
E = 3.6 kcal/mol
a
. 1.5 x 10 s
5 –1
36 bb
E = 7.3 kcal/mol
a
.
s
2 x 10 –1 –1
37 . E = 16.3 kcal/mol cc
a
.
(Continued)