Page 1015 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1015
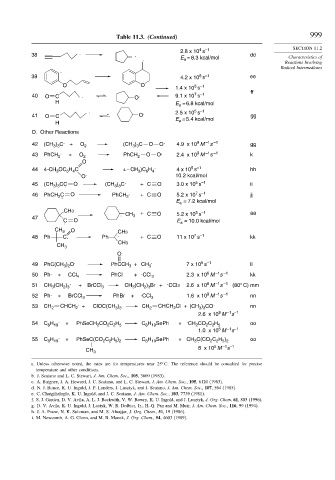
Table 11.3. (Continued) 999
SECTION 11.2
4 –1
38 . . 2.8 x 10 s dd Characteristics of
. E = 8.3 kcal/mol
a
Reactions Involving
Radical Intermediates
.
8 –1
39 4.2 x 10 s ee
O O 5 –1
1.4 x 10 s
ff
7 –1
40 O C . O . 9.1 x 10 s
H E = 6.8 kcal/mol
a
. . 2.5 x 10 s
5 –1
41 O C O E = 5.4 kcal/mol gg
H a
D. Other Reactions
9
–1 –1
42 (CH ) C . + O 2 (CH ) C O O . 4.9 x 10 M s gg
3 3
3 3
9
–1 –1
43 PhCH 2 . + O 2 PhCH 2 O O . 2.4 x 10 M s k
O
5 –1
44 4-CH OC H C 4 – CH )C H . 4 x 10 s hh
3
6 4
6 4
3
O . 10.2 kcal/mol
5 –1
45 (CH ) CC . O (CH ) C . + C O 3.0 x 10 s ii
3 3
3 3
7 –1
46 PhCH C . O PhCH 2 . + C O 5.2 x 10 s jj
2
E = 7.2 kcal/mol
a
CH3 5 –1
. CH 3 + C O 5.2 x 10 s aa
47
C O E = 10.0 kcal/mol
. a
CH 3 O CH3
7 –1
48 Ph C. Ph . +C O 11 x 10 s kk
CH3
CH 3
O
5 –1
49 PhC(CH ) O . PhCCH 3 +CH 3 . 7 x 10 s ll
3 2
–1 –1
6
50 Ph . + CCl 4 PhCl + . CCl 3 2.3 x 10 M s kk
. . 8 –1 –1
51 CH (CH ) + BrCCl 3 CH (CH ) Br + CCl3 2.6 x 10 M s (80° C) mm
3
3
2 3
2 3
–1 –1
9
52 Ph . + BrCCl 3 PhBr + . CCl 3 1.6 x 10 M s nn
53 CH 2 CHCH 2 . + ClOC(CH ) CH 2 CHCH Cl + (CH ) CO . nn
2
3 3
3 3
9
–1 –1
2.6 x 10 M s
54 C H . + PhSeCH CO C H C H SePh + . CH CO C H oo
2
2
2 2 5
2 2 5
8 19
8 19
–1 –1
5
1.0 x 10 M s
.
55 C H + PhSeC(CO C H ) C H SePh + CH C(CO C H ) oo
2 2 5 2
2 2 5 2
8 19
8 19
3 .
5
–1 –1
CH 3 8 x 10 M s
a. Unless otherwise noted, the rates are for temperatures near 25 C. The reference should be consulted for precise
temperature and other conditions.
b. J. Scaiano and L. C. Stewart, J. Am. Chem. Soc., 105, 3609 (1983).
c. A. Baignee, J. A. Howard, J. C. Scaiano, and L. C. Stewart, J. Am. Chem. Soc., 105, 6120 (1983).
d. N. J. Bunce, K. U. Ingold, J. P. Landers, J. Lusztyk, and J. Scaiano, J. Am. Chem. Soc., 107, 564 (1985).
e. C. Chatgilialoglu, K. U. Ingold, and J. C. Scaiano, J. Am. Chem. Soc., 103, 7739 (1981).
f. S. J. Garden, D. V. Avila, A. L. J. Beckwith, V. W. Bowry, K. U. Ingold, and J. Lusztyk, J. Org. Chem. 61, 805 (1996).
g. D. V. Avila, K. U. Ingold, J. Lustyk, W. R. Dolbier, Jr., H.-Q. Pan and M. Muir, J. Am. Chem. Soc., 116, 99 (1994).
h. J. A. Franz, N. K. Suleman, and M. S. Alnajjar, J. Org. Chem., 51, 19 (1986).
i. M. Newcomb, A. G. Glenn, and M. B. Manek, J. Org. Chem., 54, 4603 (1989).