Page 1018 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1018
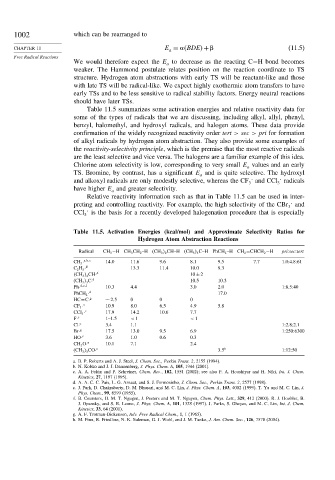
1002 which can be rearranged to
CHAPTER 11 E = BDE
+ (11.5)
a
Free Radical Reactions
We would therefore expect the E to decrease as the reacting C−H bond becomes
a
weaker. The Hammond postulate relates position on the reaction coordinate to TS
structure. Hydrogen atom abstractions with early TS will be reactant-like and those
with late TS will be radical-like. We expect highly exothermic atom transfers to have
early TSs and to be less sensitive to radical stability factors. Energy neutral reactions
should have later TSs.
Table 11.5 summarizes some activation energies and relative reactivity data for
some of the types of radicals that we are discussing, including alkyl, allyl, phenyl,
benzyl, halomethyl, and hydroxyl radicals, and halogen atoms. These data provide
confirmation of the widely recognized reactivity order tert > sec > pri for formation
of alkyl radicals by hydrogen atom abstraction. They also provide some examples of
the reactivity-selectivity principle, which is the premise that the most reactive radicals
are the least selective and vice versa. The halogens are a familiar example of this idea.
Chlorine atom selectivity is low, corresponding to very small E values and an early
a
TS. Bromine, by contrast, has a significant E and is quite selective. The hydroxyl
a
.
.
and alkoxyl radicals are only modestly selective, whereas the CF and CCl radicals
3
3
have higher E and greater selectivity.
a
Relative reactivity information such as that in Table 11.5 can be used in inter-
.
preting and controlling reactivity. For example, the high selectivity of the CBr 3 and
.
CCl 3 is the basis for a recently developed halogenation procedure that is especially
Table 11.5. Activation Energies (kcal/mol) and Approximate Selectivity Ratios for
Hydrogen Atom Abstraction Reactions
Radical CH 3 −HCH 3 CH 2 –H (CH 3
2 CH–H (CH 3
3 C–H PhCH 2 –H CH 2 =CHCH 2 −H pri:sec:tert
a b c
CH 3 · 14.0 11.6 9.6 8.1 9.5 7.7 1.0:4.8:61
d 13.3 11.4 10.0 9.3
C 2 H 5 ·
d
CH 3
2 CH· 10±2
d 10.5 10.3
(CH 3
3 C·
d e f
Ph· 10.3 4.4 3.0 2.0 1:8.5:40
d 17.0
PhCH 2 ·
g
HC≡C· ∼ 2 5 0 0 0
a
CF 3 · 10.9 8.0 6.5 4.9 5.8
c
CCl 3 · 17.9 14.2 10.6 7.7
c
F· 1–1.5 < 1 < 1
c
Cl· 3.4 1.1 1:2.8:2.1
g
Br· 17.5 13.0 9.5 6.9 1:250:6300
c 3.6 1.0 0.6 0.3
HO·
a
CH 3 O· 10.1 7.1 2.4
c 3 5 h 1:12:50
(CH 3
3 CO·
a. B. P. Roberts and A. J. Steel, J. Chem. Soc., Perkin Trans. 2, 2155 (1994).
b. N. Kobko and J. J. Dannenberg, J. Phys. Chem. A, 105, 1944 (2001).
c. A. A. Fokin and P. Schreiner, Chem. Rev., 102, 1551 (2002); see also P. A. Hooshiyar and H. Niki, Int. J. Chem.
Kinetics, 27, 1197 (1995).
d. A. A. C. C. Pais, L. G. Arnaut, and S. J. Formosinho, J. Chem. Soc., Perkin Trans. 2, 2577 (1998).
e. J. Park, D. Chakraborty, D. M. Bhusari, and M. C. Lin, J. Phys. Chem. A, 103, 4002 (1999). T. Yu and M. C. Lin, J.
Phys. Chem., 99, 8599 (1955).
f. B. Ceursters, H. M. T. Ngugen, J. Peeters and M. T. Nguyen, Chem. Phys. Lett., 329, 412 (2000). R. J. Hoobler, B.
J. Opansky, and S. R. Leone, J. Phys. Chem. A, 101, 1338 (1997). J. Parks, S. Gheyas, and M. C. Lin, Int. J. Chem.
Kinetics, 33, 64 (2001).
g. A. F. Trotman-Dickenson, Adv. Free Radical Chem., 1, 1 (1965).
h. M. Finn, R. Friedline, N. K. Suleman, G. J. Wohl, and J. M. Tanko, J. Am. Chem. Soc., 126, 7578 (2004).