Page 1142 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1142
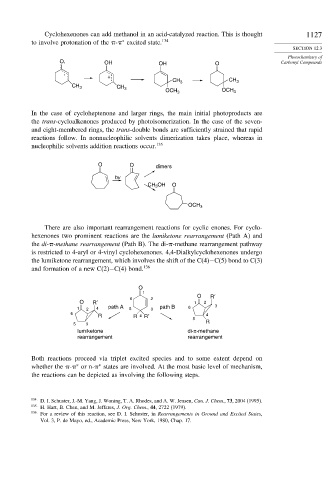
Cyclohexenones can add methanol in an acid-catalyzed reaction. This is thought 1127
∗
to involve protonation of the - excited state. 134
SECTION 12.3
Photochemistry of
O. OH OH O Carbonyl Compounds
.
+
CH 3 CH 3
CH 3
CH 3
OCH 3 OCH 3
In the case of cycloheptenone and larger rings, the main initial photoproducts are
the trans-cycloalkenones produced by photoisomerization. In the case of the seven-
and eight-membered rings, the trans-double bonds are sufficiently strained that rapid
reactions follow. In nonnucleophilic solvents dimerization takes place, whereas in
nucleophilic solvents addition reactions occur. 135
O O dimers
hv
CH 3 OH O
OCH 3
There are also important rearrangement reactions for cyclic enones. For cyclo-
hexenones two prominent reactions are the lumiketone rearrangement (Path A) and
the di- -methane rearrangement (Path B). The di- -methane rearrangement pathway
is restricted to 4-aryl or 4-vinyl cyclohexenones. 4,4-Dialkylcyclohexenones undergo
the lumiketone rearrangement, which involves the shift of the C(4)−C(5) bond to C(3)
and formation of a new C(2)−C(4) bond. 136
O
1
O R′
6 2
O R′ 1 2
1 2 4 path A 5 3 path B 6 3
6
R R 4 R′ 4
5
5 3 R
lumiketone di-π-methane
rearrangement rearrangement
Both reactions proceed via triplet excited species and to some extent depend on
whether the - or n- states are involved. At the most basic level of mechanism,
∗
∗
the reactions can be depicted as involving the following steps.
134 D. I. Schuster, J.-M. Yang, J. Woning, T. A. Rhodes, and A. W. Jensen, Can. J. Chem., 73, 2004 (1995).
135 H. Hart, B. Chen, and M. Jeffares, J. Org. Chem., 44, 2722 (1979).
136
For a review of this reaction, see D. I. Schuster, in Rearrangements in Ground and Excited States,
Vol. 3, P. de Mayo, ed., Academic Press, New York, 1980, Chap. 17.