Page 1143 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1143
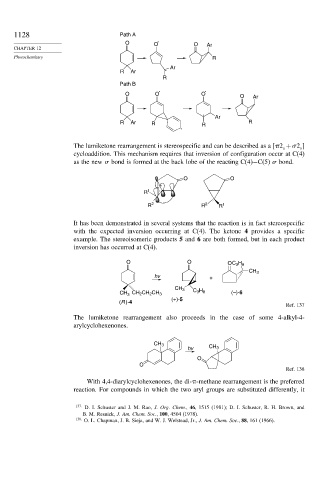
1128 Path A
O O O Ar
CHAPTER 12
Photochemistry R
Ar
R Ar
R
Path B
O O O O Ar
Ar
R Ar R R R
The lumiketone rearrangement is stereospecific and can be described as a [ 2 + 2 ]
a
a
cycloaddition. This mechanism requires that inversion of configuration occur at C(4)
as the new bond is formed at the back lobe of the reacting C(4)−C(5) bond.
O O
R 1
R 2 R 2 R 1
It has been demonstrated in several systems that the reaction is in fact stereospecific
with the expected inversion occurring at C(4). The ketone 4 provides a specific
example. The stereoisomeric products 5 and 6 are both formed, but in each product
inversion has occurred at C(4).
O O OC 3 H 8
CH 3
hv +
CH 3
C 3 H 8 (–)-6
CH 3 CH 2 CH 2 CH 3
(+)-5
(R )-4
Ref. 137
The lumiketone rearrangement also proceeds in the case of some 4-alkyl-4-
arylcyclohexenones.
CH 3
hv CH 3
O
O
Ref. 138
With 4,4-diarylcyclohexenones, the di- -methane rearrangement is the preferred
reaction. For compounds in which the two aryl groups are substituted differently, it
137 D. I. Schuster and J. M. Rao, J. Org. Chem., 46, 1515 (1981); D. I. Schuster, R. H. Brown, and
B. M. Resnick, J. Am. Chem. Soc., 100, 4504 (1978).
138
O. L. Chapman, J. B. Sieja, and W. J. Welstead, Jr., J. Am. Chem. Soc., 88, 161 (1966).