Page 1145 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1145
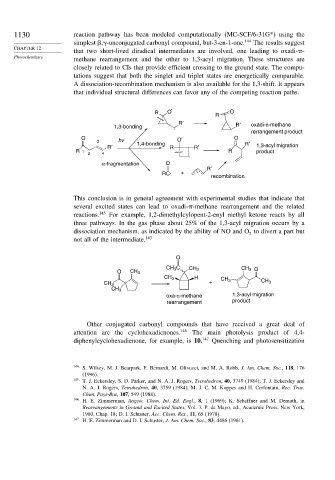
1130 reaction pathway has been modeled computationally (MC-SCF/6-31G*) using the
simplest
, -unconjugated carbonyl compound, but-3-en-1-one. 144 The results suggest
CHAPTER 12
that two short-lived diradical intermediates are involved, one leading to oxadi- -
Photochemistry
methane rearrangement and the other to 1,3-acyl migration. These structures are
closely related to CIs that provide efficient crossing to the ground state. The compu-
tations suggest that both the singlet and triplet states are energetically comparable.
A dissociation-recombination mechanism is also available for the 1,3-shift. It appears
that individual structural differences can favor any of the competing reaction paths.
R O O
R
R′ R′
1,3-bonding oxadi-π-methane
rerrangement product
O O O
3 hv 1,4-bonding R′
R′ R R′ 1,3-acyl migration
R 1 R product
2 4
α-fragmentation O
R′
RC +
recombination
This conclusion is in general agreement with experimental studies that indicate that
several excited states can lead to oxadi- -methane rearrangement and the related
reactions. 145 For example, 1,2-dimethylcylopent-2-enyl methyl ketone reacts by all
three pathways. In the gas phase about 25% of the 1,3-acyl migration occurs by a
dissociation mechanism, as indicated by the ability of NO and O to divert a part but
2
not all of the intermediate. 143
O
CH 3 C CH 3 O
O CH 3 CH 3
H
CH 3 CH 3
+ CH 3
CH 3
CH 3
oxa-π-methane 1,3-acyl migration
rearrangement product
Other conjugated carbonyl compounds that have received a great deal of
attention are the cyclohexadienones. 146 The main photolysis product of 4,4-
diphenylcyclohexadienone, for example, is 10. 147 Quenching and photosensitization
144
S. Wilsey, M. J. Bearpark, F. Bernardi, M. Olivucci, and M. A. Robb, J. Am. Chem. Soc., 118, 176
(1996).
145
T. J. Eckersley, S. D. Parker, and N. A. J. Rogers, Tetrahedron, 40, 3749 (1984); T. J. Eckersley and
N. A. J. Rogers, Tetrahedron, 40, 3759 (1984); M. J. C. M. Koppes and H. Cerfontain, Rec. Trav.
Chim. Pays-Bas, 107, 549 (1988).
146 H. E. Zimmerman, Angew. Chem. Int. Ed. Engl., 8, 1 (1969); K. Schaffner and M. Demuth, in
Rearrangements in Ground and Excited States, Vol. 3, P. de Mayo, ed., Academic Press, New York,
1980, Chap. 18; D. I. Schuster, Acc. Chem. Res., 11, 65 (1978).
147
H. E. Zimmerman and D. I. Schuster, J. Am. Chem. Soc., 83, 4486 (1961).