Page 471 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 471
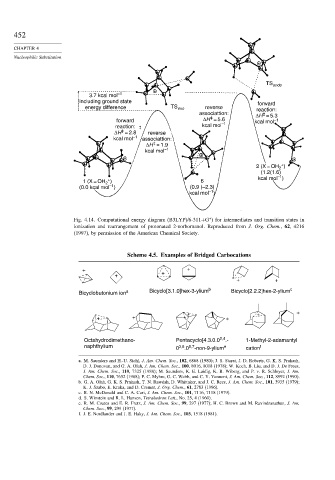
452
CHAPTER 4
Nucleophilic Substitution
TS endo
3.7 kcal mol –1
including ground state forward
energy difference TS exo reverse reaction:
associattion: ΔH = 5.3
‡
‡
forward ΔH = 5.6 kcal mol –1
kcal mol –1
reaction: ‡
‡
ΔH = 2.8 reverse
kcal mol –1 associattion:
‡
ΔH = 1.9
kcal mol –1
+
2 (X = OH )
2
(1.2(1.6)
–1
+
1 (X = OH ) 6 kcal mol )
2
–1
(0.0 kcal mol ) (0.9 (–2.3)
–1
kcal mol )
∗
Fig. 4.14. Computational energy diagram (B3LYP)/6-311+G ) for intermediates and transition states in
ionization and rearrangement of protonated 2-norbornanol. Reproduced from J. Org. Chem., 62, 4216
(1997), by permission of the American Chemical Society.
Scheme 4.5. Examples of Bridged Carbocations
+ + +
+
+ +
Bicyclobutonium ion a Bicyclo[3.1.0]hex-3-ylium b Bicyclo[2.2.2]hex-2-ylium c
+ + +
+ + +
Octahydrodimethano- Pentacyclo[4.3.0.0 2,4 .- 1-Methyl-2-adamantyl
naphthylium 0 3,8 .0 5,7 -non-9-ylium e cation f
a. M. Saunders and H.-U. Siehl, J. Am. Chem. Soc., 102, 6868 (1980); J. S. Starat, J. D. Roberts, G. K. S. Prakash,
D. J. Donovan, and G. A. Olah, J. Am. Chem. Soc., 100, 8016, 8018 (1978); W. Koch, B. Liu, and D. J. De Frees,
J. Am. Chem. Soc., 110, 7325 (1988); M. Saunders, K. E. Laidig, K. B. Wiberg, and P. v. R. Schleyer, J. Am.
Chem. Soc., 110, 7652 (1988); P. C. Myhre, G. C. Webb, and C. Y. Yannoni, J. Am. Chem. Soc., 112, 8992 (1990).
b. G. A. Olah, G. K. S. Prakash, T. N. Rawdah, D. Whittaker, and J. C. Rees, J. Am. Chem. Soc., 101, 3935 (1979);
K. J. Szabo, E. Kraka, and D. Cremer, J. Org. Chem., 61, 2783 (1996).
c. R. N. McDonald and C. A. Curi, J. Am. Chem. Soc., 101, 7116, 7118 (1979).
d. S. Winstein and R. L. Hansen, Tetrahedron Lett., No. 25, 4 (1960).
e. R. M. Coates and E. R. Fretz, J. Am. Chem. Soc., 99, 297 (1977); H. C. Brown and M. Ravindranathan, J. Am.
Chem. Soc., 99, 299 (1977).
f. J. E. Nordlander and J. E. Haky, J. Am. Chem. Soc., 103, 1518 (1981).