Page 477 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 477
458 Similar reactions are observed over other strongly acidic catalysts, such as AlCl and
3
SbF . 169 The acidic catalysts also promote dimerization and oligomerization of alkenes
5
CHAPTER 4 by mechanisms that are well known in the solution chemistry of carbocations. 170
Nucleophilic Substitution
CH 3
H + CH 3 + CH 3
C CH 2 (CH ) C + H C (CH ) CCH C
2
3 3
2
3 3
CH
CH 3 3 CH 3
+ CH 3
(CH ) CCH C (CH ) CCH CH(CH ) + (CH ) CCH C(CH )
2
3 3
3 3
3 3
3 2
2
3 2
CH 3
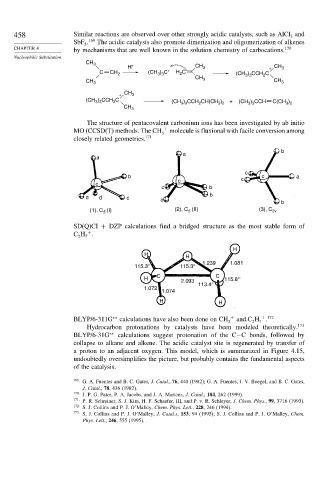
The structure of pentacovalent carbonium ions has been investigated by ab initio
MO (CCSD(T) methods. The CH + molecule is fluxional with facile conversion among
5
closely related geometries. 171
b
a
a
c
b c a
c c
c
c b
b
d d c
c
b
(I) (2), C (II) (3), C 2v
(1), C 5 5
SD(Q)CI + DZP calculations find a bridged structure as the most stable form of
+
C H .
2 7
H
H
H
1.239 1.081
115.3° 115.3°
C C
H 2.093 115.8°
113.4°
1.072
1.074
H H
+ 172
BLYP/6-311G ∗∗ calculations have also been done on CH 5 + and.C H .
7
2
Hydrocarbon protonations by catalysts have been modeled theoretically. 173
BLYP/6-31G ∗∗ calculations suggest protonation of the C−C bonds, followed by
collapse to alkane and alkene. The acidic catalyst site is regenerated by transfer of
a proton to an adjacent oxygen. This model, which is summarized in Figure 4.15,
undoubtedly oversimplifies the picture, but probably contains the fundamental aspects
of the catalysis.
169 G. A. Fuentes and B. C. Gates, J. Catal., 76, 440 (1982); G. A. Fuentes, J. V. Boegel, and B. C. Gates,
J. Catal., 78, 436 (1982).
170 J. P. G. Pater, P. A. Jacobs, and J. A. Martens, J. Catal., 184, 262 (1999).
171
P. R. Schreiner, S. J. Kim, H. F. Schaefer, III, and P. v. R. Schleyer, J. Chem. Phys., 99, 3716 (1993).
172 S. J. Collins and P. J. O’Malley, Chem. Phys. Lett., 228, 246 (1994).
173
S. J. Collins and P. J. O’Malley, J. Catal.s, 153, 94 (1995); S. J. Collins and P. J. O’Malley, Chem.
Phys. Lett., 246, 555 (1995).