Page 832 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 832
newly attached electrophile or the substituent is eliminated from the intermediate on 815
rearomatization.
SECTION 9.4
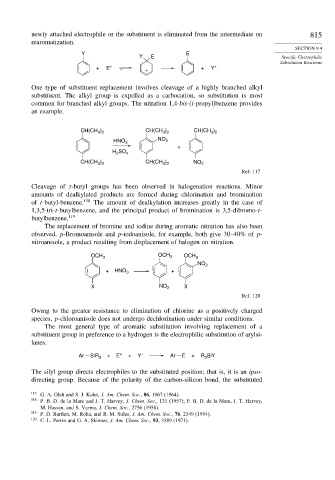
Y E
Y E Specific Electrophilic
Substitution Reactions
+ E + + + Y +
One type of substituent replacement involves cleavage of a highly branched alkyl
substituent. The alkyl group is expelled as a carbocation, so substitution is most
common for branched alkyl groups. The nitration 1,4-bis-(i-propyl)benzene provides
an example.
) CH(CH ) CH(CH )
CH(CH 3 2 3 2 3 2
HNO 3 NO 2
+
H SO 4
2
CH(CH ) CH(CH ) NO 2
3 2
3 2
Ref. 117
Cleavage of t-butyl groups has been observed in halogenation reactions. Minor
amounts of dealkylated products are formed during chlorination and bromination
of t-butyl-benzene. 118 The amount of dealkylation increases greatly in the case of
1,3,5-tri-t-butylbenzene, and the principal product of bromination is 3,5-dibromo-t-
butylbenzene. 119
The replacement of bromine and iodine during aromatic nitration has also been
observed. p-Bromoanisole and p-iodoanisole, for example, both give 30–40% of p-
nitroanisole, a product resulting from displacement of halogen on nitration.
OCH 3 OCH 3 OCH 3
NO 2
+ HNO 3 +
X NO 2 X
Ref. 120
Owing to the greater resistance to elimination of chlorine as a positively charged
species, p-chloroanisole does not undergo dechlorination under similar conditions.
The most general type of aromatic substitution involving replacement of a
substituent group in preference to a hydrogen is the electrophilic substitution of arylsi-
lanes.
Ar SiR 3 + E + + Y – Ar E + R SiY
3
The silyl group directs electrophiles to the substituted position; that is, it is an ipso-
directing group. Because of the polarity of the carbon-silicon bond, the substituted
117 G. A. Olah and S. J. Kuhn, J. Am. Chem. Soc., 86, 1067 (1964).
118
P. B. D. de la Mare and J. T. Harvey, J. Chem. Soc., 131 (1957); P. B. D. de la Mare, J. T. Harvey,
M. Hassan, and S. Varma, J. Chem. Soc., 2756 (1958).
119 P. D. Bartlett, M. Roha, and R. M. Stiles, J. Am. Chem. Soc., 76, 2349 (1954).
120
C. L. Perrin and G. A. Skinner, J. Am. Chem. Soc., 93, 3389 (1971).