Page 833 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 833
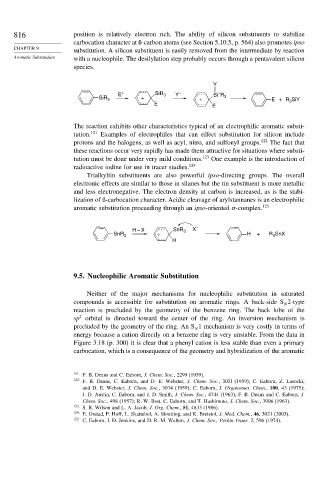
816 position is relatively electron rich. The ability of silicon substituents to stabilize
carbocation character at ß-carbon atoms (see Section 5.10.5, p. 564) also promotes ipso
CHAPTER 9
substitution. A silicon substituent is easily removed from the intermediate by reaction
Aromatic Substitution with a nucleophile. The desilylation step probably occurs through a pentavalent silicon
species.
Y
–
E + SiR 3 Y – Si R
SiR 3 + + 3 E +R SiY
E 3
E
The reaction exhibits other characteristics typical of an electrophilic aromatic substi-
tution. 121 Examples of electrophiles that can effect substitution for silicon include
protons and the halogens, as well as acyl, nitro, and sulfonyl groups. 122 The fact that
these reactions occur very rapidly has made them attractive for situations where substi-
tution must be done under very mild conditions. 123 One example is the introduction of
radioactive iodine for use in tracer studies. 124
Trialkyltin substituents are also powerful ipso-directing groups. The overall
electronic effects are similar to those in silanes but the tin substituent is more metallic
and less electronegative. The electron density at carbon is increased, as is the stabi-
lization of ß-carbocation character. Acidic cleavage of arylstannanes is an electrophilic
aromatic substitution proceeding through an ipso-oriented -complex. 125
H – X SnR 3 X –
SnR 3 + H + R 3 SnX
H
9.5. Nucleophilic Aromatic Substitution
Neither of the major mechanisms for nucleophilic substitution in saturated
compounds is accessible for substitution on aromatic rings. A back-side S 2-type
N
reaction is precluded by the geometry of the benzene ring. The back lobe of the
2
sp orbital is directed toward the center of the ring. An inversion mechanism is
precluded by the geometry of the ring. An S 1 mechanism is very costly in terms of
N
energy because a cation directly on a benzene ring is very unstable. From the data in
Figure 3.18 (p. 300) it is clear that a phenyl cation is less stable than even a primary
carbocation, which is a consequence of the geometry and hybridization of the aromatic
121 F. B. Deans and C. Eaborn, J. Chem. Soc., 2299 (1959).
122
F. B. Deans, C. Eaborn, and D. E. Webster, J. Chem. Soc., 303l (1959); C. Eaborn, Z. Lasocki,
and D. E. Webster, J. Chem. Soc., 3034 (1959); C. Eaborn, J. Organomet. Chem., 100, 43 (1975);
J. D. Austin, C. Eaborn, and J. D. Smith, J. Chem. Soc., 4744 (1963); F. B. Deans and C. Eaborn, J.
Chem. Soc., 498 (1957); R. W. Bott, C. Eaborn, and T. Hashimoto, J. Chem. Soc., 3906 (1963).
123 S. R. Wilson and L. A. Jacob, J. Org. Chem., 51, 4833 (1986).
124 E. Orstad, P. Hoff, L. Skattebol, A. Skretting, and K. Breistol, J. Med. Chem., 46, 3021 (2003).
125
C. Eaborn, I. D. Jenkins, and D. R. M. Walton, J. Chem. Soc., Perkin Trans. 2, 596 (1974).