Page 835 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 835
818 usually the dominant factor, so the order of reactivity of the halogens is I > Br > Cl > F.
In nucleophilic aromatic substitution, the formation of the addition intermediate is
CHAPTER 9 usually the rate-determining step, so the ease of C−X bond breaking does not affect
Aromatic Substitution the rate. When this is the case, the order of reactivity is often F > Cl > Br > I. 127
This order is the result of the polar effect of the halogen. The stronger bond dipoles
associated with the more electronegative halogens favor the addition step and thus
increase the overall rates of reaction.
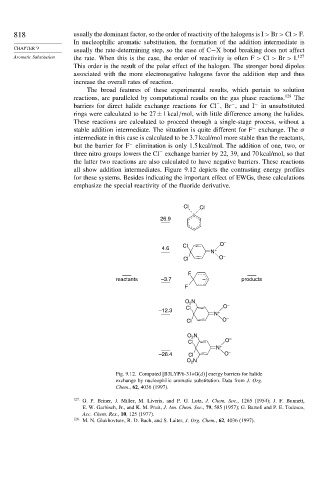
The broad features of these experimental results, which pertain to solution
reactions, are paralleled by computational results on the gas phase reactions. 128 The
−
barriers for direct halide exchange reactions for Cl ,Br , and I − in unsubstituted
−
rings were calculated to be 27±1kcal/mol, with little difference among the halides.
These reactions are calculated to proceed through a single-stage process, without a
−
stable addition intermediate. The situation is quite different for F exchange. The
intermediate in this case is calculated to be 3.7 kcal/mol more stable than the reactants,
−
but the barrier for F elimination is only 1.5 kcal/mol. The addition of one, two, or
−
three nitro groups lowers the Cl exchange barrier by 22, 39, and 70 kcal/mol, so that
the latter two reactions are also calculated to have negative barriers. These reactions
all show addition intermediates. Figure 9.12 depicts the contrasting energy profiles
for these systems. Besides indicating the important effect of EWGs, these calculations
emphasize the special reactivity of the fluoride derivative.
Cl Cl
26.9
Cl O –
4.6
N +
Cl O –
F
reactants –3.7 – products
F
O 2 N –
Cl O
–12.3
N +
Cl O –
O N –
2
Cl O
N +
–26.4 Cl O –
O N
2
Fig. 9.12. Computed [B3LYP/6-31+G(d ] energy barriers for halide
exchange by nucleophilic aromatic substitution. Data from J. Org.
Chem., 62, 4036 (1997).
127 G. P. Briner, J. Miller, M. Liveris, and P. G. Lutz, J. Chem. Soc., 1265 (1954); J. F. Bunnett,
E. W. Garbisch, Jr., and K. M. Pruit, J. Am. Chem. Soc., 79, 585 (1957); G. Bartoli and P. E. Todesco,
Acc. Chem. Res., 10, 125 (1977).
128
M. N. Glukhovtsev, R. D. Bach, and S. Laiter, J. Org. Chem., 62, 4036 (1997).