Page 838 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 838
such as a nitro group but do not require a halide or other leaving group. The reactions 821
proceed through addition intermediates. 146
SECTION 9.5
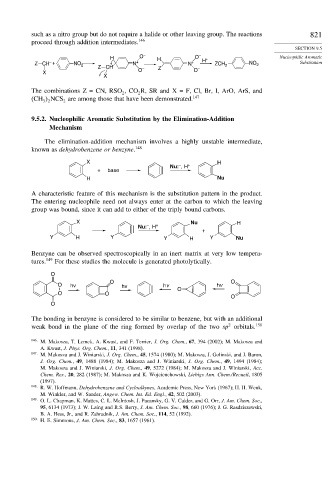
H O – H O – H + Nucleophilic Aromatic
Z CH – + NO 2 Z CH N + N + ZCH 2 NO 2 Substitution
X O – Z O –
X
The combinations Z = CN, RSO ,CO R, SR and X = F, Cl, Br, I, ArO, ArS, and
2 2
(CH NCS are among those that have been demonstrated. 147
3 2 2
9.5.2. Nucleophilic Aromatic Substitution by the Elimination-Addition
Mechanism
The elimination-addition mechanism involves a highly unstable intermediate,
known as dehydrobenzene or benzyne. 148
X H
–
Nu: , H +
+ base
H Nu
A characteristic feature of this mechanism is the substitution pattern in the product.
The entering nucleophile need not always enter at the carbon to which the leaving
group was bound, since it can add to either of the triply bound carbons.
X Nu H
–
Nu: , H +
+
Y H Y Y H Y Nu
Benzyne can be observed spectroscopically in an inert matrix at very low tempera-
tures. 149 For these studies the molecule is generated photolytically.
O
O O
O hv hv hv hv
O
O O
O
O
The bonding in benzyne is considered to be similar to benzene, but with an additional
2
weak bond in the plane of the ring formed by overlap of the two sp orbitals. 150
146
M. Makosza, T. Lemek, A. Kwast, and F. Terrier, J. Org. Chem., 67, 394 (2002); M. Makosza and
A. Kwast, J. Phys. Org. Chem., 11, 341 (1998).
147 M. Makosza and J. Winiarski, J. Org. Chem., 45, 1574 (1980); M. Makosza, J. Golinski, and J. Baron,
J. Org. Chem., 49, 1488 (1984); M. Makosza and J. Winiarski, J. Org. Chem., 49, 1494 (1984);
M. Makosza and J. Winiarski, J. Org. Chem., 49, 5272 (1984); M. Makosza and J. Winiarski, Acc.
Chem. Res., 20, 282 (1987); M. Makosza and K. Wojcienchowski, Liebigs Ann. Chem./Recueil, 1805
(1997).
148
R. W. Hoffmann, Dehydrobenzene and Cycloalkynes, Academic Press, New York (1967); H. H. Wenk,
M. Winkler, and W. Sander, Angew. Chem. Int. Ed. Engl., 42, 502 (2003).
149 O. L. Chapman, K. Mattes, C. L. McIntosh, J. Pacansky, G. V. Calder, and G. Orr, J. Am. Chem. Soc.,
95, 6134 (1973); J. W. Laing and R.S. Berry, J. Am. Chem. Soc., 98, 660 (1976); J. G. Rasdziszewski,
B. A. Hess, Jr., and R. Zahradnik, J. Am. Chem. Soc., 114, 52 (1992).
150
H. E. Simmons, J. Am. Chem. Soc., 83, 1657 (1961).