Page 839 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 839
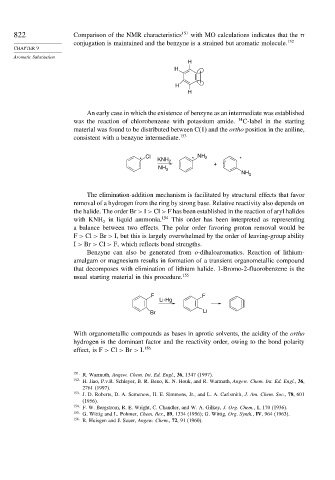
822 Comparison of the NMR characteristics 151 with MO calculations indicates that the
conjugation is maintained and the benzyne is a strained but aromatic molecule. 152
CHAPTER 9
Aromatic Substitution
H
H
H
H
An early case in which the existence of benzyne as an intermediate was established
was the reaction of chlorobenzene with potassium amide. 14 C-label in the starting
material was found to be distributed between C(1) and the ortho position in the aniline,
consistent with a benzyne intermediate. 153
Cl NH 2
* KNH 2 * *
+
NH 3
NH 2
The elimination-addition mechanism is facilitated by structural effects that favor
removal of a hydrogen from the ring by strong base. Relative reactivity also depends on
the halide. The order Br > I > Cl > F has been established in the reaction of aryl halides
with KNH in liquid ammonia. 154 This order has been interpreted as representing
2
a balance between two effects. The polar order favoring proton removal would be
F > Cl > Br > I, but this is largely overwhelmed by the order of leaving-group ability
I > Br > Cl > F, which reflects bond strengths.
Benzyne can also be generated from o-dihaloaromatics. Reaction of lithium-
amalgam or magnesium results in formation of a transient organometallic compound
that decomposes with elimination of lithium halide. 1-Bromo-2-fluorobenzene is the
usual starting material in this procedure. 155
F F
Li-Hg
Br Li
With organometallic compounds as bases in aprotic solvents, the acidity of the ortho
hydrogen is the dominant factor and the reactivity order, owing to the bond polarity
effect, is F > Cl > Br > I. 156
151 R. Warmuth, Angew. Chem. Int. Ed. Engl., 36, 1347 (1997).
152
H. Jiao, P.v.R. Schleyer, B. R. Beno, K. N. Houk, and R. Warmuth, Angew. Chem. Int. Ed. Engl., 36,
2761 (1997).
153
J. D. Roberts, D. A. Semenow, H. E. Simmons, Jr., and L. A. Carlsmith, J. Am. Chem. Soc., 78, 601
(1956).
154 F. W. Bergstrom, R. E. Wright, C. Chandler, and W. A. Gilkey, J. Org. Chem., 1, 170 (1936).
155 G. Wittig and L. Pohmer, Chem. Ber., 89, 1334 (1956); G. Wittig, Org. Synth., IV, 964 (1963).
156
R. Huisgen and J. Sauer, Angew. Chem., 72, 91 (1960).