Page 840 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 840
Addition of nucleophiles such as ammonia or alcohols or their conjugate bases to 823
benzynes takes place very rapidly. These nucleophilic additions are believed to involve
capture of the nucleophile, followed by protonation to give the substituted benzene. 157 SECTION 9.5
Nucleophilic Aromatic
Substitution
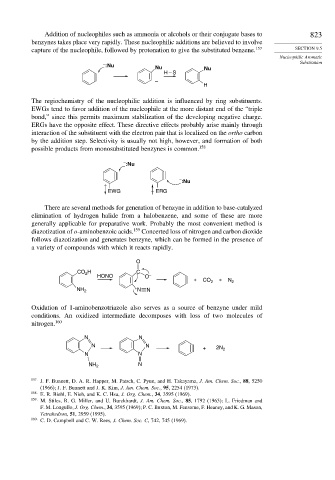
– :Nu Nu Nu
H – S
–
H
The regiochemistry of the nucleophilic addition is influenced by ring substituents.
EWGs tend to favor addition of the nucleophile at the more distant end of the “triple
bond,” since this permits maximum stabilization of the developing negative charge.
ERGs have the opposite effect. These directive effects probably arise mainly through
interaction of the substituent with the electron pair that is localized on the ortho carbon
by the addition step. Selectivity is usually not high, however, and formation of both
possible products from monosubstituted benzynes is common. 158
– :Nu
– :Nu
EWG ERG
There are several methods for generation of benzyne in addition to base-catalyzed
elimination of hydrogen halide from a halobenzene, and some of these are more
generally applicable for preparative work. Probably the most convenient method is
diazotization of o-aminobenzoic acids. 159 Concerted loss of nitrogen and carbon dioxide
follows diazotization and generates benzyne, which can be formed in the presence of
a variety of compounds with which it reacts rapidly.
O
CO H C
2
HONO O –
+ CO 2 + N 2
NH 2 + N N
Oxidation of 1-aminobenzotriazole also serves as a source of benzyne under mild
conditions. An oxidized intermediate decomposes with loss of two molecules of
nitrogen. 160
N N
N N
+ 2N 2
N N
N
NH 2
157
J. F. Bunnett, D. A. R. Happer, M. Patsch, C. Pyun, and H. Takayama, J. Am. Chem. Soc., 88, 5250
(1966); J. F. Bunnett and J. K. Kim, J. Am. Chem. Soc., 95, 2254 (1973).
158 E. R. Biehl, E. Nieh, and K. C. Hsu, J. Org. Chem., 34, 3595 (1969).
159 M. Stiles, R. G. Miller, and U. Burckhardt, J. Am. Chem. Soc., 85, 1792 (1963); L. Friedman and
F. M. Longullo, J. Org. Chem., 34, 3595 (1969); P. C. Buxton, M. Fensome, F. Heaney, and K. G. Mason,
Tetrahedron, 51, 2959 (1995).
160
C. D. Campbell and C. W. Rees, J. Chem. Soc. C, 742, 745 (1969).