Page 841 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 841
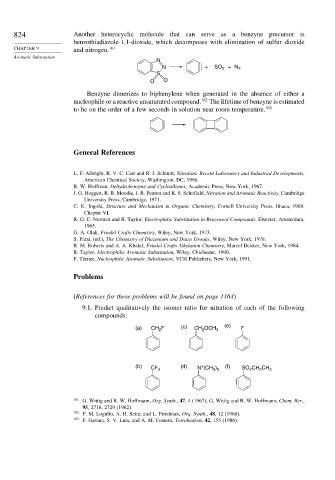
824 Another heterocyclic molecule that can serve as a benzyne precursor is
benzothiadiazole-1,1-dioxide, which decomposes with elimination of sulfur dioxide
CHAPTER 9 and nitrogen. 161
Aromatic Substitution
N
N + SO 2 +N 2
S
O O
Benzyne dimerizes to biphenylene when generated in the absence of either a
nucleophile or a reactive unsaturated compound. 162 The lifetime of benzyne is estimated
to be on the order of a few seconds in solution near room temperature. 163
General References
L. F. Albright, R. V. C. Carr and R. J. Schmitt, Nitration: Recent Laboratory and Industrial Developments,
American Chemical Society, Washington, DC, 1996.
R. W. Hoffman, Dehydrobenzene and Cycloalkynes, Academic Press, New York, 1967.
J. G. Hoggett, R. B. Moodie, J. R. Penton and K. S. Schofield, Nitration and Aromatic Reactivity, Cambridge
University Press, Cambridge, 1971.
C. K. Ingold, Structure and Mechanism in Organic Chemistry, Cornell University Press, Ithaca, 1969,
Chapter VI.
R. O. C. Norman and R. Taylor, Electrophilic Substitution in Benzenoid Compounds, Elsevier, Amsterdam,
1965.
G. A. Olah, Friedel Crafts Chemistry, Wiley, New York, 1973.
S. Patai, (ed.), The Chemistry of Diazonium and Diazo Groups, Wiley, New York, 1978.
R. M. Roberts and A. A. Khalaf, Friedel-Crafts Alkylation Chemistry, Marcel Dekker, New York, 1984.
R. Taylor, Electrophilic Aromatic Substitution, Wiley, Chichester, 1990.
F. Terrier, Nucleophilic Aromatic Substitution, VCH Publishers, New York, 1991.
Problems
(References for these problems will be found on page 1164)
9.1. Predict qualitatively the isomer ratio for nitration of each of the following
compounds:
(a) CH F (c) CH OCH 3 (e) F
2
2
(b) CF 3 (d) N (CH ) (f) SO CH CH 3
+
3 3
2
2
161 G. Wittig and R. W. Hoffmann, Org. Synth., 47, 4 (1967); G. Wittig and R. W. Hoffmann, Chem. Ber.,
95, 2718, 2729 (1962).
162
F. M. Logullo, A. H. Seitz, and L. Friedman, Org. Synth., 48, 12 (1968).
163
F. Gavina, S. V. Luis, and A. M. Costero, Tetrahedron, 42, 155 (1986).