Page 843 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 843
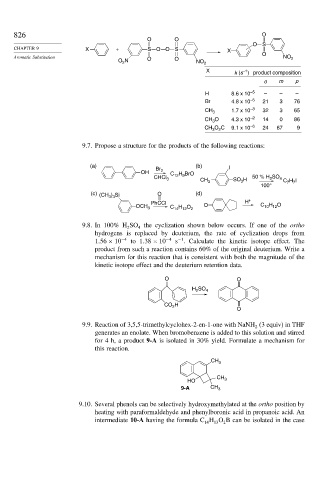
826 O
O O
O S
CHAPTER 9 X + SO O S X
Aromatic Substitution O O O NO 2
O N NO 2
2
X k (s ) product composition
–1
o m p
H 8.6 x 10 –5 – – –
Br 4.8 x 10 –5 21 3 76
CH 3 1.7 x 10 –3 32 3 65
CH O 4.3 x 10 –2 14 0 86
3
CH 3 O 2 C 9.1 x 10 –6 24 67 9
9.7. Propose a structure for the products of the following reactions:
(a) Br (b) I
OH 2 C H BrO
CHCl 3 12 9 CH SO H 50 % H SO 4 C H I
2
3
3
100° 7 7
(c) (CH ) Si O (d)
3 3
PhCCl H +
OCH 3 C H O 2 O C H O
10 12
14 12
9.8. In 100% H SO the cyclization shown below occurs. If one of the ortho
4
2
hydrogens is replaced by deuterium, the rate of cyclization drops from
−1
1 56 × 10 −4 to 1 38 × 10 −4 s . Calculate the kinetic isotope effect. The
product from such a reaction contains 60% of the original deuterium. Write a
mechanism for this reaction that is consistent with both the magnitude of the
kinetic isotope effect and the deuterium retention data.
O O
H 2 SO 4
CO H
2
O
9.9. Reaction of 3,5,5-trimethylcyclohex-2-en-1-one with NaNH (3 equiv) in THF
2
generates an enolate. When bromobenzene is added to this solution and stirred
for 4 h, a product 9-A is isolated in 30% yield. Formulate a mechanism for
this reaction.
CH 3
CH
HO 3
9-A CH 3
9.10. Several phenols can be selectively hydroxymethylated at the ortho position by
heating with paraformaldehyde and phenylboronic acid in propanoic acid. An
intermediate 10-A having the formula C H O B can be isolated in the case
14
2
13