Page 846 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 846
c. A very polar EWG that does not have -conjugation capacity, e.g., 829
+
N CH .
3 3
PROBLEMS
d. A group without strong -conjugating capacity that is less electronegative
than carbon, e.g., Si CH .
3 3
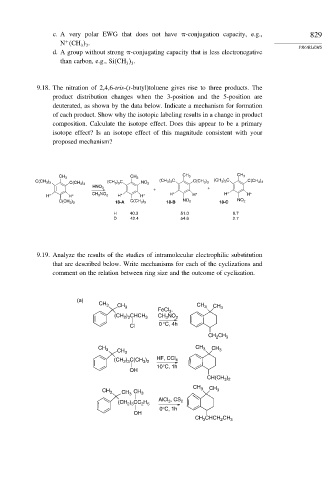
9.18. The nitration of 2,4,6-tris-(t-butyl)toluene gives rise to three products. The
product distribution changes when the 3-position and the 5-position are
deuterated, as shown by the data below. Indicate a mechanism for formation
of each product. Show why the isotopic labeling results in a change in product
composition. Calculate the isotope effect. Does this appear to be a primary
isotope effect? Is an isotope effect of this magnitude consistent with your
proposed mechanism?
CH 3 CH 3
CH 3 CH 3
C(CH 3 ) 3 (CH 3 ) 3 C NO 2 (CH 3 ) 3 C C(CH 3 ) 3 (CH 3 ) 3 C C(CH 3 ) 3
C(CH 3 ) 3
HNO 3 +
+
H* H* CH 3 NO 2 H* H* H* H* H* H*
C(CH 3 ) 3 18-A C(CH 3 ) 3 18-B NO 2 18-C NO 2
H 40.3 51.0 8.7
D 42.4 54.6 2.7
9.19. Analyze the results of the studies of intramolecular electrophilic substitution
that are described below. Write mechanisms for each of the cyclizations and
comment on the relation between ring size and the outcome of cyclization.
(a)
CH 3 CH CH CH
3
FeCl , 3 3
3
(CH ) CHCH 3 CH 3 NO 2
2 3
Cl 0 °C, 4h
CH CH 3
2
CH
CH 3 3 CH
CH 3 3
(CH ) C(CH ) HF, CCl 4
3 2
2 3
10°C, 1h
OH
CH(CH )
3 2
CH 3
CH 3 CH 3 CH 3 CH 3
AlCl , CS
(CH ) CC H 3 2
2 5
2 3
0°C, 1h
OH
CHCH CH
CH 3 2 3