Page 847 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 847
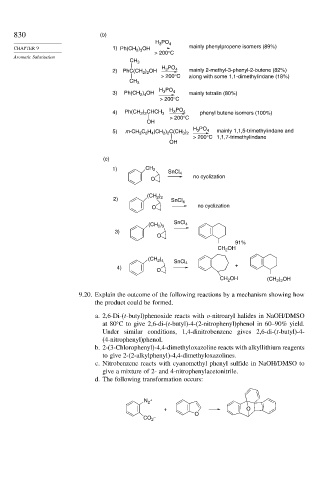
830 (b)
H PO 4
3
CHAPTER 9 1) Ph(CH ) OH mainly phenylpropene isomers (89%)
2 3
> 200°C
Aromatic Substitution
CH 3
H PO
2) PhC(CH ) OH 3 4 mainly 2-methyl-3-phenyl-2-butene (82%)
2 2
> 200°C along with some 1,1-dimethylindane (18%)
CH 3
H PO
3) Ph(CH ) OH 3 4 mainly tetralin (80%)
2 4
> 200°C
4) Ph(CH ) CHCH 3 H 3 PO 4 phenyl butene isomers (100%)
2 2
> 200°C
OH
H PO
5) m-CH 3 6 4 2 2 3 2 3 4 mainly 1,1,5-trimethylindane and
C H (CH ) C(CH )
> 200°C 1,1,7-trimethylindane
OH
(c)
1) CH 2
SnCl 4
O no cyclization
)
(CH 2 2
2) SnCl 4
O no cyclization
SnCl
(CH ) 4
2 3
3)
O
91%
OH
CH 2
(CH )
2 4
SnCl 4
4) +
O
CH OH (CH 2 2
) OH
2
9.20. Explain the outcome of the following reactions by a mechanism showing how
the product could be formed.
a. 2,6-Di-(t-butyl)phenoxide reacts with o-nitroaryl halides in NaOH/DMSO
at 80 C to give 2,6-di-(t-butyl)-4-(2-nitrophenyl)phenol in 60–90% yield.
Under similar conditions, 1,4-dinitrobenzene gives 2,6-di-(t-butyl)-4-
(4-nitrophenyl)phenol.
b. 2-(3-Chlorophenyl)-4,4-dimethyloxazoline reacts with alkyllithium reagents
to give 2-(2-alkylphenyl)-4,4-dimethyloxazolines.
c. Nitrobenzene reacts with cyanomethyl phenyl sulfide in NaOH/DMSO to
give a mixture of 2- and 4-nitrophenylacetonitrile.
d. The following transformation occurs:
N 2 +
+ O
O
CO 2 –