Page 848 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 848
e. Reaction of benzene with 3,3,3-trifluoropropene in the presence of BF 831
3
gives 3,3,3-trifluoropropylbenzene.
PROBLEMS
f. 3-Chloronitrobenzene reacts with 4-amino-1,2,4-triazole in K · O–t-Bu/
+ −
DMSO to give 2-chloro-4-nitroaniline.
g. Good yields of tetralone can be obtained from 4-phenylbutanoic acid or
the corresponding acyl chloride in the presence of the strongly acidic resin
Nafion-H. With 3-phenylpropanonic acid, only the acyl chloride gives a
cyclization product.
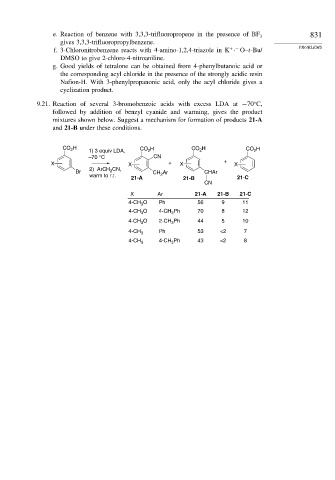
9.21. Reaction of several 3-bromobenzoic acids with excess LDA at −70 C,
followed by addition of benzyl cyanide and warming, gives the product
mixtures shown below. Suggest a mechanism for formation of products 21-A
and 21-B under these conditions.
CO H 1) 3 equiv LDA, CO 2 H CO 2 H CO 2 H
2
–70 °C CN
X X + X + X
CN,
Br 2) ArCH 2 CH 2 Ar CHAr
warm to r.t.
21-A 21-B 21-C
CN
X Ar 21-A 21-B 21-C
4-CH O Ph 56 9 11
3
O 4-CH Ph 70 8 12
4-CH 3 3
4-CH O 2-CH Ph 44 5 10
3
3
4-CH 3 Ph 53 <2 7
4-CH Ph 43 <2 8
4-CH 3 3