Page 850 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 850
4
834 pericyclic reactions. These various approaches conclude that TSs with certain orbital
alignments are energetically favorable (allowed), whereas others lead to high-energy
CHAPTER 10
(forbidden) TSs. The stabilized TSs share certain electronic features with aromatic
Concerted Pericyclic systems, whereas the high-energy TSs are more similar to antiaromatic systems. 4b c As
Reactions
we will see shortly, this leads to rules similar to the Hückel and Mobius relationships for
aromaticity (see Section 8.1) that allow prediction of the outcome of the reactions on the
basis of the properties of the orbitals of the reactants. Because these reactions proceed
through highly ordered cyclic transition structures with specific orbital alignments, the
concerted pericyclic reactions often have characteristic and predictable stereochemistry.
In many cases, the reactions exhibit regioselectivity that can be directly related to the
effect of orbital interactions on TS structure. Similarly, substituent effects on reactivity
can be interpreted in terms of the effect of the substituents on the interacting orbitals.
A great deal of effort has been expended to model the transition structures of
5
concerted pericyclic reactions. All of the major theoretical approaches, semiempirical
MO, ab initio MO, and DFT have been applied to the problem and some comparisons
6
have been made. The conclusions drawn generally parallel the orbital symmetry rules
in their prediction of reactivity and stereochemistry and provide additional insight into
substituent effects.
We discuss several categories of concerted pericyclic reactions, including Diels-
Alder and other cycloaddition reactions, electrocyclic reactions, and sigmatropic
rearrangements. The common feature is a concerted mechanism involving a cyclic TS
with continuous electronic reorganization. The fundamental aspects of these reactions
can be analyzed in terms of orbital symmetry characteristics associated with the TS.
For each major group of reactions, we examine how regio- and stereoselectivity are
determined by the cyclic TS.
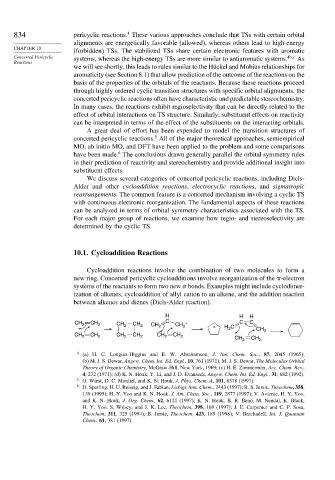
10.1. Cycloaddition Reactions
Cycloaddition reactions involve the combination of two molecules to form a
new ring. Concerted pericyclic cycloadditions involve reorganization of the -electron
systems of the reactants to form two new bonds. Examples might include cyclodimer-
ization of alkenes, cycloaddition of allyl cation to an alkene, and the addition reaction
between alkenes and dienes (Diels-Alder reaction).
H H H
CH 2 CH 2 CH 2 CH 2 CH 2 C CH 2 + + H C C C
2
CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH CH 2
2
4
(a) H. C. Longuet-Higgins and E. W. Abrahamson, J. Am. Chem. Soc., 87, 2045 (1965);
(b) M. J. S. Dewar, Angew. Chem. Int. Ed. Engl., 10, 761 (1971); M. J. S. Dewar, The Molecular Orbital
Theory of Organic Chemistry, McGraw-Hill, New York, 1969; (c) H. E. Zimmerman, Acc. Chem. Res.,
4, 272 (1971); (d) K. N. Houk, Y. Li, and J. D. Evanseck, Angew. Chem. Int. Ed. Engl., 31, 682 (1992).
5 O. Wiest, D. C. Montiel, and K. N. Houk, J. Phys. Chem. A, 101, 8378 (1997).
6
D. Sperling, H. U. Reissig, and J. Fabian, Liebigs Ann. Chem., 2443 (1997); B. S. Jursic, Theochem, 358,
139 (1995); H.-Y. Yoo and K. N. Houk, J. Am. Chem. Soc., 119, 2877 (1997); V. Aviente, H. Y, Yoo,
and K. N. Houk, J. Org. Chem., 62, 6121 (1997); K. N. Houk, B. R. Beno, M. Nendal, K. Black,
H. Y. Yoo, S. Wilsey, and J. K. Lee, Theochem, 398, 169 (1997); J. E. Carpenter and C. P. Sosa,
Theochem, 311, 325 (1994); B. Jursic, Theochem, 423, 189 (1998); V. Brachadell, Int. J. Quantum
Chem., 61, 381 (1997).