Page 851 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 851
The cycloadditions can be characterized by specifying the number of electrons 835
involved for each species, and for the above three cases, this would be 2+2 , 2+2 ,
and 2 + 4 , respectively. Some such reactions occur readily, whereas others are not SECTION 10.1
observed. We will learn, for example, that of the three reactions above, only the Cycloaddition Reactions
alkene-diene cycloaddition occurs readily. The pattern of reactivity can be understood
by application of the principle of conservation of orbital symmetry.
The most important of the concerted cycloaddition reactions is the Diels-Alder
reaction between a diene and an alkene derivative to form a cyclohexene. The alkene
reactant usually has a substituent and is called the dienophile. We discuss this reaction
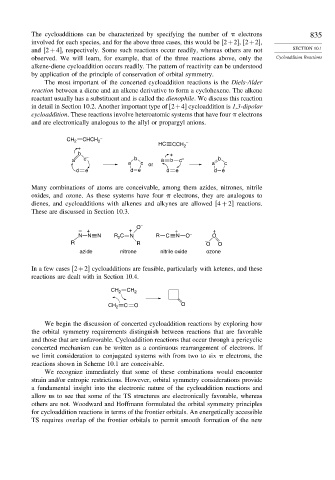
in detail in Section 10.2. Another important type of 2+4 cycloaddition is 1,3-dipolar
cycloaddition. These reactions involve heteroatomic systems that have four electrons
and are electronically analogous to the allyl or propargyl anions.
CH 2 CHCH 2 –
HC CCH –
+ 2
b +
a c – b a b c – b
a c or a c
d e d e d e d e
Many combinations of atoms are conceivable, among them azides, nitrones, nitrile
oxides, and ozone. As these systems have four electrons, they are analogous to
dienes, and cycloadditions with alkenes and alkynes are allowed 4 + 2 reactions.
These are discussed in Section 10.3.
O –
– + + + +
N N N R 2 C N R C N O – O
R R – O O
azide nitrone nitrile oxide ozone
In a few cases 2+2 cycloadditions are feasible, particularly with ketenes, and these
reactions are dealt with in Section 10.4.
CH 2 CH 2
CH 2 C O O
We begin the discussion of concerted cycloaddition reactions by exploring how
the orbital symmetry requirements distinguish between reactions that are favorable
and those that are unfavorable. Cycloaddition reactions that occur through a pericyclic
concerted mechanism can be written as a continuous rearrangement of electrons. If
we limit consideration to conjugated systems with from two to six electrons, the
reactions shown in Scheme 10.1 are conceivable.
We recognize immediately that some of these combinations would encounter
strain and/or entropic restrictions. However, orbital symmetry considerations provide
a fundamental insight into the electronic nature of the cycloaddition reactions and
allow us to see that some of the TS structures are electronically favorable, whereas
others are not. Woodward and Hoffmann formulated the orbital symmetry principles
for cycloaddition reactions in terms of the frontier orbitals. An energetically accessible
TS requires overlap of the frontier orbitals to permit smooth formation of the new