Page 854 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 854
838
CHAPTER 10 ψ ψ ψ
π π* ψ 1 2 3 4
Concerted Pericyclic symmetric (S) antisymmetric (A) symmetric (S) antisymmetric(A) symmetric (S) antisymmetric(A)
Reactions
ethene orbitals butadiene orbitals
σ σ' σ* σ'* π π ∗
symmetric (S) antisymmetric (A) symmetric (S) antisymmetric(A) symmetric (S) antisymmetric(A)
cyclohexene orbitals
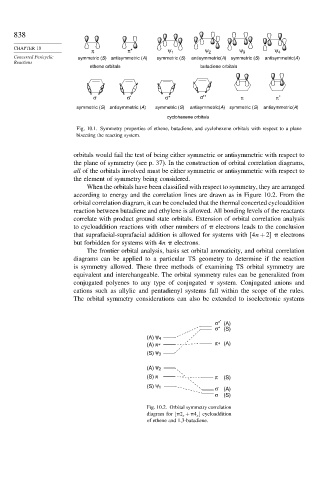
Fig. 10.1. Symmetry properties of ethene, butadiene, and cyclohexene orbitals with respect to a plane
bisecting the reacting system.
orbitals would fail the test of being either symmetric or antisymmetric with respect to
the plane of symmetry (see p. 37). In the construction of orbital correlation diagrams,
all of the orbitals involved must be either symmetric or antisymmetric with respect to
the element of symmetry being considered.
When the orbitals have been classified with respect to symmetry, they are arranged
according to energy and the correlation lines are drawn as in Figure 10.2. From the
orbital correlation diagram, it can be concluded that the thermal concerted cycloaddition
reaction between butadiene and ethylene is allowed. All bonding levels of the reactants
correlate with product ground state orbitals. Extension of orbital correlation analysis
to cycloaddition reactions with other numbers of electrons leads to the conclusion
that suprafacial-suprafacial addition is allowed for systems with 4n+2 electrons
but forbidden for systems with 4n electrons.
The frontier orbital analysis, basis set orbital aromaticity, and orbital correlation
diagrams can be applied to a particular TS geometry to determine if the reaction
is symmetry allowed. These three methods of examining TS orbital symmetry are
equivalent and interchangeable. The orbital symmetry rules can be generalized from
conjugated polyenes to any type of conjugated system. Conjugated anions and
cations such as allylic and pentadienyl systems fall within the scope of the rules.
The orbital symmetry considerations can also be extended to isoelectronic systems
σ* ' (A)
σ* (S)
(A) ψ 4
(A) π∗ π∗ (A)
(S) ψ 3
(A) ψ 2
(S) π π (S)
(S) ψ 1
σ ' (A)
σ (S)
Fig. 10.2. Orbital symmetry correlation
diagram for [ 2 s + 4 s ] cycloaddition
of ethene and 1,3-butadiene.