Page 855 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 855
containing heteroatoms. Thus the C=C double bonds can be replaced by C=N, C=O, 839
C=S, N=O, N=N, and other related multiple bonds.
SECTION 10.2
The Diels-Alder Reaction
10.2. The Diels-Alder Reaction
10.2.1. Stereochemistry of the Diels-Alder Reaction
The [ 4 + 2 ] cycloaddition of alkenes and dienes is a very useful method
s
s
for forming substituted cyclohexenes. This reaction is known as the Diels-Alder
7
(abbreviated D-A in this chapter) reaction. The transition structure for a concerted
reaction requires that the diene adopt the s-cis conformation. The diene and substituted
alkene (called the dienophile) approach each other in approximately parallel planes.
This reaction has been the object of extensive mechanistic and computational study, as
well as synthetic application. For most systems, the reactivity pattern, regioselectivity,
and stereoselectivity are consistent with a concerted process. In particular, the reaction
is a stereospecific syn (suprafacial) addition with respect to both the alkene and the
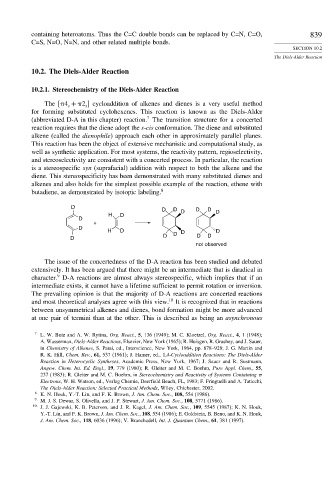
diene. This stereospecificity has been demonstrated with many substituted dienes and
alkenes and also holds for the simplest possible example of the reaction, ethene with
butadiene, as demonstrated by isotopic labeling. 8
D D D D D
H D D D
D
+
D
H D D D
D D D D
D
not observed
The issue of the concertedness of the D-A reaction has been studied and debated
extensively. It has been argued that there might be an intermediate that is diradical in
9
character. D-A reactions are almost always stereospecific, which implies that if an
intermediate exists, it cannot have a lifetime sufficient to permit rotation or inversion.
The prevailing opinion is that the majority of D-A reactions are concerted reactions
and most theoretical analyses agree with this view. 10 It is recognized that in reactions
between unsymmetrical alkenes and dienes, bond formation might be more advanced
at one pair of termini than at the other. This is described as being an asynchronous
7 L. W. Butz and A. W. Rytina, Org. React., 5, 136 (1949); M. C. Kloetzel, Org. React., 4, 1 (1948);
A. Wasserman, Diels-Alder Reactions, Elsevier, New York (1965); R. Huisgen, R. Grashey, and J. Sauer,
in Chemistry of Alkenes, S. Patai, ed., Interscience, New York, 1964, pp. 878–928; J. G. Martin and
R. K. Hill, Chem. Rev., 61, 537 (1961); J. Hamer, ed., 1,4-Cycloaddition Reactions: The Diels-Alder
Reaction in Heterocyclic Syntheses, Academic Press, New York, 1967; J. Sauer and R. Sustmann,
Angew. Chem. Int. Ed. Engl., 19, 779 (1980); R. Gleiter and M. C. Boehm, Pure Appl. Chem., 55,
237 (1983); R. Gleiter and M. C. Boehm, in Stereochemistry and Reactivity of Systems Containing
Electrons, W. H. Watson, ed., Verlag Chemie, Deerfield Beach, FL, 1983; F. Fringuelli and A. Taticchi,
The Diels-Alder Reaction: Selected Practical Methods, Wiley, Chichester, 2002.
8
K. N. Houk, Y.-T. Lin, and F. K. Brown, J. Am. Chem. Soc., 108, 554 (1986).
9 M. J. S. Dewar, S. Olivella, and J. P. Stewart, J. Am. Chem. Soc., 108, 5771 (1986).
10
J. J. Gajewski, K. B. Peterson, and J. R. Kagel, J. Am. Chem. Soc., 109, 5545 (1987); K. N. Houk,
Y.-T. Lin, and F. K. Brown, J. Am. Chem. Soc., 108, 554 (1986); E. Goldstein, B. Beno, and K. N. Houk,
J. Am. Chem. Soc., 118, 6036 (1996); V. Branchadell, Int. J. Quantum Chem., 61, 381 (1997).