Page 857 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 857
841
SECTION 10.2
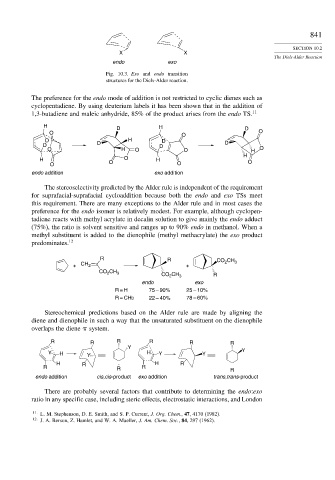
X X
The Diels-Alder Reaction
endo exo
Fig. 10.3. Exo and endo transition
structures for the Diels-Alder reaction.
The preference for the endo mode of addition is not restricted to cyclic dienes such as
cyclopentadiene. By using deuterium labels it has been shown that in the addition of
1,3-butadiene and maleic anhydride, 85% of the product arises from the endo TS. 11
H D H D
O O O
D H D
D D D D
O H O O H O
H
H O H
O O O O
endo addition exo addition
The stereoselectivity predicted by the Alder rule is independent of the requirement
for suprafacial-suprafacial cycloaddition because both the endo and exo TSs meet
this requirement. There are many exceptions to the Alder rule and in most cases the
preference for the endo isomer is relatively modest. For example, although cyclopen-
tadiene reacts with methyl acrylate in decalin solution to give mainly the endo adduct
(75%), the ratio is solvent sensitive and ranges up to 90% endo in methanol. When a
methyl substituent is added to the dienophile (methyl methacrylate) the exo product
predominates. 12
R R CO CH
+ CH 2 + 2 3
CH
CO 2 3 CO CH 3 R
2
endo exo
R = H 75 – 90% 25 – 10%
R = CH3 22 – 40% 78 – 60%
Stereochemical predictions based on the Alder rule are made by aligning the
diene and dienophile in such a way that the unsaturated substituent on the dienophile
overlaps the diene system.
R R R R R R
Y
Y H Y H Y Y Y
H R H R
R R R R
endo addition cis,cis-product exo addition trans,trans-product
There are probably several factors that contribute to determining the endo:exo
ratio in any specific case, including steric effects, electrostatic interactions, and London
11 L. M. Stephenson, D. E. Smith, and S. P. Current, J. Org. Chem., 47, 4170 (1982).
12
J. A. Berson, Z. Hamlet, and W. A. Mueller, J. Am. Chem. Soc., 84, 297 (1962).