Page 860 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 860
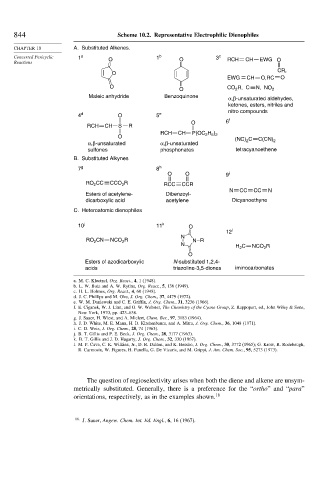
844 Scheme 10.2. Representative Electrophilic Dienophiles
CHAPTER 10 A. Substituted Alkenes.
Concerted Pericyclic 1 a O 1 b O 3 c RCH CH EWG O
Reactions
CR,
O
EWG = CH O,RC O
O R, C N, NO
O CO 2 2
Maleic anhydride Benzoquinone
α,β-unsaturated aldehydes,
ketones, esters, nitriles and
nitro compounds
4 d O 5 e
O 6 f
RCH CH S R
RCH CH P(OC H )
O 2 5 2
(NC) C C(CN) 2
2
α,β-unsaturated α,β-unsaturated
sulfones phosphonates tetracyanoethene
B. Substituted Alkynes
7 g 8 h
O O 9 i
RO CC CCO R RCC CCR
2
2
N CC CC N
Esters of acetylene- Dibenzoyl-
dicarboxylic acid acetylene Dicyanoethyne
C. Heteroatomic dienophiles
10 j 11 k O
12 l
N
RO CN NCO R N R
2
2
N H C NCO R
2
2
O
Esters of azodicarboxylic N-substituted 1,2,4-
acids triazoline-3,5-diones iminocarbonates
a. M. C. Kloetzel, Org. React., 4, 1 (1948).
b. L. W. Butz and A. W. Rytina, Org. React., 5, 136 (1949).
c. H. L. Holmes, Org. React., 4, 60 (1948).
d. J. C. Phillips and M. Oku, J. Org. Chem., 37, 4479 (1972).
e. W. M. Daniewski and C. E. Griffin, J. Org. Chem., 31, 3236 (1966).
f. E. Ciganek, W. J. Linn, and O. W. Webster, The Chemistry of the Cyano Group, Z. Rappoport, ed., John Wiley & Sons,
New York, 1970, pp. 423–638.
g. J. Sauer, H. Wiest, and A. Mielert, Chem. Ber., 97, 3183 (1964).
h. J. D. White, M. E. Mann, H. D. Kirshenbaum, and A. Mitra, J. Org. Chem., 36, 1048 (1971).
i. C. D. Weis, J. Org. Chem., 28, 74 (1963).
j. B. T. Gillis and P. E. Beck, J. Org. Chem., 28, 3177 (1963).
k. B. T. Gillis and J. D. Hagarty, J. Org. Chem., 32, 330 (1967).
l. M. P. Cava, C. K. Wilkins, Jr., D. R. Dalton, and K. Bessho, J. Org. Chem., 30, 3772 (1965); G. Krow, R. Rodebaugh,
R. Carmosin, W. Figures, H. Panella, G. De Vicaris, and M. Grippi, J. Am. Chem. Soc., 95, 5273 (1973).
The question of regioselectivity arises when both the diene and alkene are unsym-
metrically substituted. Generally, there is a preference for the “ortho” and “para”
orientations, respectively, as in the examples shown. 18
18
J. Sauer, Angew. Chem. Int. Ed. Engl., 6, 16 (1967).