Page 861 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 861
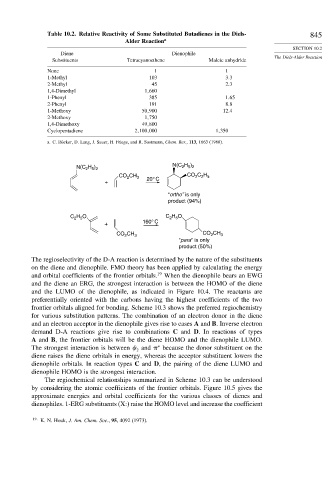
Table 10.2. Relative Reactivity of Some Substituted Butadienes in the Diels- 845
Alder Reaction a
SECTION 10.2
Diene Dienophile
The Diels-Alder Reaction
Substituents Tetracyanoethene Maleic anhydride
None 1 1
1-Methyl 103 3 3
2-Methyl 45 2 3
1,4-Dimethyl 1 660
1-Phenyl 385 1 65
2-Phenyl 191 8 8
1-Methoxy 50 900 12 4
2-Methoxy 1 750
1,4-Dimethoxy 49 800
Cyclopentadiene 2 100 000 1 350
a. C. Rücker, D. Lang, J. Sauer, H. Friege, and R. Sustmann, Chem. Ber., 113, 1663 (1980).
N(C H ) N(C H )
2 5 2
2 5 2
CO CH 3 CO C H
2 2 5
2
+ 20° C
“ortho” is only
product (94%)
C H O C H O
2 5
2 5
+ 160° C
CO CH 3 CO CH 3
2
2
“para” is only
product (50%)
The regioselectivity of the D-A reaction is determined by the nature of the substituents
on the diene and dienophile. FMO theory has been applied by calculating the energy
and orbital coefficients of the frontier orbitals. 19 When the dienophile bears an EWG
and the diene an ERG, the strongest interaction is between the HOMO of the diene
and the LUMO of the dienophile, as indicated in Figure 10.4. The reactants are
preferentially oriented with the carbons having the highest coefficients of the two
frontier orbitals aligned for bonding. Scheme 10.3 shows the preferred regiochemistry
for various substitution patterns. The combination of an electron donor in the diene
and an electron acceptor in the dienophile gives rise to cases A and B. Inverse electron
demand D-A reactions give rise to combinations C and D. In reactions of types
A and B, the frontier orbitals will be the diene HOMO and the dienophile LUMO.
∗
The strongest interaction is between
and because the donor substituent on the
2
diene raises the diene orbitals in energy, whereas the acceptor substituent lowers the
dienophile orbitals. In reaction types C and D, the pairing of the diene LUMO and
dienophile HOMO is the strongest interaction.
The regiochemical relationships summarized in Scheme 10.3 can be understood
by considering the atomic coefficients of the frontier orbitals. Figure 10.5 gives the
approximate energies and orbital coefficients for the various classes of dienes and
dienophiles. 1-ERG substituents (X:) raise the HOMO level and increase the coefficient
19
K. N. Houk, J. Am. Chem. Soc., 95, 4092 (1973).