Page 862 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 862
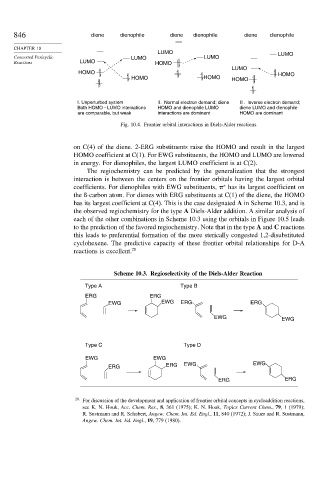
846 diene dienophile diene dienophile diene dienophile
CHAPTER 10
LUMO LUMO
Concerted Pericyclic LUMO LUMO
Reactions LUMO HOMO
LUMO
HOMO HOMO
HOMO HOMO HOMO
I. Unperturbed system II. Normal electron demand; diene III. Inverse electron demand;
Both HOMO – LUMO interactions HOMO and dienophile LUMO diene LUMO and dienophile
are comparable, but weak interactions are dominant HOMO are dominant
Fig. 10.4. Frontier orbital interactions in Diels-Alder reactions.
on C(4) of the diene. 2-ERG substituents raise the HOMO and result in the largest
HOMO coefficient at C(1). For EWG substituents, the HOMO and LUMO are lowered
in energy. For dienophiles, the largest LUMO coefficient is at C(2).
The regiochemistry can be predicted by the generalization that the strongest
interaction is between the centers on the frontier orbitals having the largest orbital
coefficients. For dienophiles with EWG substituents, has its largest coefficient on
∗
the ß-carbon atom. For dienes with ERG substituents at C(1) of the diene, the HOMO
has its largest coefficient at C(4). This is the case designated A in Scheme 10.3, and is
the observed regiochemistry for the type A Diels-Alder addition. A similar analysis of
each of the other combinations in Scheme 10.3 using the orbitals in Figure 10.5 leads
to the prediction of the favored regiochemistry. Note that in the type A and C reactions
this leads to preferential formation of the more sterically congested 1,2-disubstituted
cyclohexene. The predictive capacity of these frontier orbital relationships for D-A
reactions is excellent. 20
Scheme 10.3. Regioselectivity of the Diels-Alder Reaction
Type A Type B
ERG ERG
EWG EWG ERG ERG
EWG EWG
Type C Type D
EWG EWG
ERG ERG EWG EWG
ERG ERG
20
For discussion of the development and application of frontier orbital concepts in cycloaddition reactions,
see K. N. Houk, Acc. Chem. Res., 8, 361 (1975); K. N. Houk, Topics Current Chem., 79, 1 (1979);
R. Sustmann and R. Schubert, Angew. Chem. Int. Ed. Engl., 11, 840 (1972); J. Sauer and R. Sustmann,
Angew. Chem. Int. Ed. Engl., 19, 779 (1980).