Page 864 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 864
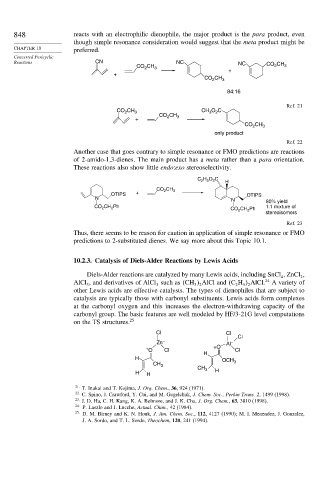
848 reacts with an electrophilic dienophile, the major product is the para product, even
though simple resonance consideration would suggest that the meta product might be
CHAPTER 10 preferred.
Concerted Pericyclic
Reactions CN NC NC CO CH
CO CH 3 2 3
2
+
+
CO CH 3
2
84:16
Ref. 21
CO CH 3 CH O C
2
2
3
CO CH
+ 2 3
CO 2 CH 3
only product
Ref. 22
Another case that goes contrary to simple resonance or FMO predictions are reactions
of 2-amido-1,3-dienes. The main product has a meta rather than a para orientation.
These reactions also show little endo:exo stereoselectivity.
C 2 H 5 O 2 C
H
CO 2 CH 3
OTIPS + OTIPS
N N 80% yield
CO 2 CH 2 Ph 1:1 mixture of
CO 2 CH 2 Ph
stereoisomers
Ref. 23
Thus, there seems to be reason for caution in application of simple resonance or FMO
predictions to 2-substituted dienes. We say more about this Topic 10.1.
10.2.3. Catalysis of Diels-Alder Reactions by Lewis Acids
Diels-Alder reactions are catalyzed by many Lewis acids, including SnCl , ZnCl ,
2
4
AlCl , and derivatives of AlCl such as CH AlCl and C H AlCl. 24 A variety of
5 2
3 2
3
3
2
other Lewis acids are effective catalysts. The types of dienophiles that are subject to
catalysis are typically those with carbonyl substituents. Lewis acids form complexes
at the carbonyl oxygen and this increases the electron-withdrawing capacity of the
carbonyl group. The basic features are well modeled by HF/3-21G level computations
on the TS structures. 25
Cl Cl
Cl
Zn – Al –
+O
+ O Cl Cl
H
H
OCH 3
CH 3
CH 3 H
H H
21 T. Inukai and T. Kojima, J. Org. Chem., 36, 924 (1971).
22
C. Spino, J. Crawford, Y. Cui, and M. Gugelchuk, J. Chem. Soc., Perkin Trans. 2, 1499 (1998).
23 J. D. Ha, C. H. Kang, K. A. Belmore, and J. K. Cha, J. Org. Chem., 63, 3810 (1998).
24 P. Laszlo and J. Lucche, Actual. Chim., 42 (1984).
25
D. M. Birney and K. N. Houk, J. Am. Chem. Soc., 112, 4127 (1990); M. I. Menendez, J. Gonzalez,
J. A. Sordo, and T. L. Sordo, Theochem, 120, 241 (1994).