Page 865 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 865
This complexation accentuates both the energy and orbital distortion effects of the 849
substituent and enhances both the reactivity and selectivity of the dienophile relative
26
to the uncomplexed compound. Usually, both regioselectivity and exo,endo stereose- SECTION 10.2
lectivity increase. Part of this may be due to the lower reaction temperature. However, The Diels-Alder Reaction
the catalysts also shift the reaction toward a higher degree of charge transfer by making
the EWG substituent more electrophilic.
CH 3 CH 3 CO CH 2
2
CH 3 +
+
CO CH 3 CO CH 3
2
2
“para” “meta”
Uncatalyzed reaction, 120° C, 6h 70% 30%
AlCl -catalyzed 20° C, 3h 95% 5%
3
Ref. 27
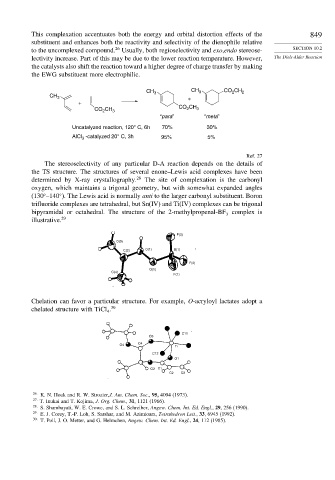
The stereoselectivity of any particular D-A reaction depends on the details of
the TS structure. The structures of several enone–Lewis acid complexes have been
determined by X-ray crystallography. 28 The site of complexation is the carbonyl
oxygen, which maintains a trigonal geometry, but with somewhat expanded angles
(130 –140 ). The Lewis acid is normally anti to the larger carbonyl substituent. Boron
trifluoride complexes are tetrahedral, but Sn(IV) and Ti(IV) complexes can be trigonal
bipyramidal or octahedral. The structure of the 2-methylpropenal-BF complex is
3
illustrative. 29
F(3)
C(3)
C(2) C(1) B(1)
F(2)
O(1)
C(4)
F(1)
Chelation can favor a particular structure. For example, O-acryloyl lactates adopt a
chelated structure with TiCl . 30
4
C11
O3
O4 C4 Ti
C12
O1
O2 C1
C2 C3
26 K. N. Houk and R. W. Strozier,J. Am. Chem. Soc., 95, 4094 (1973).
27
T. Inukai and T. Kojima, J. Org. Chem., 31, 1121 (1966).
28
S. Shambayati, W. E. Crowe, and S. L. Schreiber, Angew. Chem. Int. Ed. Engl., 29, 256 (1990).
29 E. J. Corey, T.-P. Loh, S. Sarshar, and M. Azimioara, Tetrahedron Lett., 33, 6945 (1992).
30
T. Poll, J. O. Metter, and G. Helmchen, Angew. Chem. Int. Ed. Engl., 24, 112 (1985).