Page 866 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 866
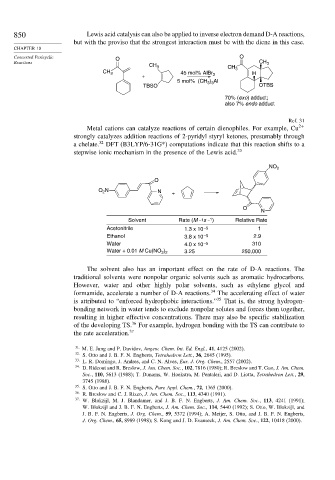
850 Lewis acid catalysis can also be applied to inverse electron demand D-A reactions,
but with the proviso that the strongest interaction must be with the diene in this case.
CHAPTER 10
Concerted Pericyclic O O
Reactions CH 3
CH 3
CH 3
CH 3 H
+ 45 mol% AlBr 3
5 mol% (CH ) Al
3 3
TBSO OTBS
70% (exo) adduct;
also 7% endo adduct
Ref. 31
Metal cations can catalyze reactions of certain dienophiles. For example, Cu 2+
strongly catalyzes addition reactions of 2-pyridyl styryl ketones, presumably through
a chelate. 32 DFT (B3LYP/6-31G*) computations indicate that this reaction shifts to a
stepwise ionic mechanism in the presence of the Lewis acid. 33
NO 2
O
N
O 2 N +
O
N
Solvent Rate (M –1s – 1 ) Relative Rate
Acetonitrile 1.3 x 10 –5 1
Ethanol 3.8 x 10 –5 2.9
Water –5 310
4.0 x 10
Water + 0.01 M Cu(NO ) 3.25 250,000
3 2
The solvent also has an important effect on the rate of D-A reactions. The
traditional solvents were nonpolar organic solvents such as aromatic hydrocarbons.
However, water and other highly polar solvents, such as ethylene glycol and
formamide, accelerate a number of D-A reactions. 34 The accelerating effect of water
is attributed to “enforced hydrophobic interactions.” 35 That is, the strong hydrogen-
bonding network in water tends to exclude nonpolar solutes and forces them together,
resulting in higher effective concentrations. There may also be specific stabilization
36
of the developing TS. For example, hydrogen bonding with the TS can contribute to
the rate acceleration. 37
31 M. E. Jung and P. Davidov, Angew. Chem. Int. Ed. Engl., 41, 4125 (2002).
32
S. Otto and J. B. F. N. Engberts, Tetrahedron Lett., 36, 2645 (1995).
33 L. R. Domingo, J. Andres, and C. N. Alves, Eur. J. Org. Chem., 2557 (2002).
34
D. Rideout and R. Breslow, J. Am. Chem. Soc., 102, 7816 (1980); R. Breslow and T. Guo, J. Am. Chem.
Soc., 110, 5613 (1988); T. Dunams, W. Hoekstra, M. Pentaleri, and D. Liotta, Tetrahedron Lett., 29,
3745 (1988).
35
S. Otto and J. B. F. N. Engberts, Pure Appl. Chem., 72, 1365 (2000).
36 R. Breslow and C. J. Rizzo, J. Am. Chem. Soc., 113, 4340 (1991).
37
W. Blokzijl, M. J. Blandamer, and J. B. F. N. Engberts, J. Am. Chem. Soc., 113, 4241 (1991);
W. Blokzijl and J. B. F. N. Engberts, J. Am. Chem. Soc., 114, 5440 (1992); S. Otto, W. Blokzijl, and
J. B. F. N. Engberts, J. Org. Chem., 59, 5372 (1994); A. Meijer, S. Otto, and J. B. F. N. Engberts,
J. Org. Chem., 65, 8989 (1998); S. Kong and J. D. Evanseck, J. Am. Chem. Soc., 122, 10418 (2000).