Page 869 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 869
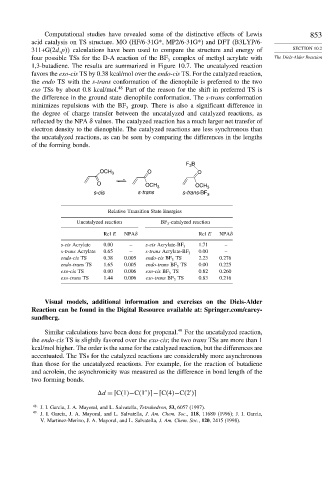
Computational studies have revealed some of the distinctive effects of Lewis 853
acid catalysis on TS structure. MO (HF/6-31G*, MP2/6-31G*) and DFT (B3LYP/6-
311+G(2d,p calculations have been used to compare the structure and energy of SECTION 10.2
four possible TSs for the D-A reaction of the BF complex of methyl acrylate with The Diels-Alder Reaction
3
1,3-butadiene. The results are summarized in Figure 10.7. The uncatalyzed reaction
favors the exo-cis TS by 0.38 kcal/mol over the endo-cis TS. For the catalyzed reaction,
the endo TS with the s-trans conformation of the dienophile is preferred to the two
exo TSs by about 0.8 kcal/mol. 48 Part of the reason for the shift in preferred TS is
the difference in the ground state dienophile conformation. The s-trans conformation
minimizes repulsions with the BF group. There is also a significant difference in
3
the degree of charge transfer between the uncatalyzed and catalyzed reactions, as
reflected by the NPA values. The catalyzed reaction has a much larger net transfer of
electron density to the dienophile. The catalyzed reactions are less synchronous than
the uncatalyzed reactions, as can be seen by comparing the differences in the lengths
of the forming bonds.
F B
3
OCH 3 O O
O
OCH 3 OCH 3
s-cis s-trans s-trans-BF 3
Relative Transition State Energies
Uncatalyzed reaction BF 3 -catalyzed reaction
Rel E NPA Rel E NPA
s-cis Acrylate 0.00 – s-cis Acrylate-BF 3 1.71 –
s-trans Acrylate 0.65 – s-trans Acrylate-BF 3 0.00 –
endo-cis TS 0.38 0.005 endo-cis BF 3 TS 2.23 0.276
endo-trans TS 1.65 0.005 endo-trans BF 3 TS 0.00 0.225
exo-cis TS 0.00 0.006 exo-cis BF 3 TS 0.82 0.260
exo-trans TS 1.44 0.006 exo-trans BF 3 TS 0.83 0.216
Visual models, additional information and exercises on the Diels-Alder
Reaction can be found in the Digital Resource available at: Springer.com/carey-
sundberg.
49
Similar calculations have been done for propenal. For the uncatalyzed reaction,
the endo-cis TS is slightly favored over the exo-cis; the two trans TSs are more than 1
kcal/mol higher. The order is the same for the catalyzed reaction, but the differences are
accentuated. The TSs for the catalyzed reactions are considerably more asynchronous
than those for the uncatalyzed reactions. For example, for the reaction of butadiene
and acrolein, the asynchronicity was measured as the difference in bond length of the
two forming bonds.
d = C 1 −C 1 − C 4 −C 2
48 J. I. Garcia, J. A. Mayoral, and L. Salvatella, Tetrahedron, 53, 6057 (1997).
49
J. I. Garcia, J. A. Mayoral, and L. Salvatella, J. Am. Chem. Soc., 118, 11680 (1996); J. I. Garcia,
V. Martinez-Merino, J. A. Mayoral, and L. Salvatella, J. Am. Chem. Soc., 120, 2415 (1998).