Page 870 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 870
854 1.393 1.393
1.372
CHAPTER 10 1.383 1.373 1.382
Concerted Pericyclic
Reactions 1.195 2.326 2.088 2.305
1.331 2.103
1.469 1.389
1.333 1.195 1.389
1.472
TS endo s-cis
TS endo s-trans
1.393
1.393
1.372
1.383 1.373
1.383
2.337
2.080 2.313
2.100
1.469 1.390 1.472
1.193 1.390
1.330
1.197
1.335
TS exo s-cis TS exo s-trans
1.356 1.403 1.397
1.365
1.394 1.389
2.661
2.531
1.248
1.908 1.986
1.243
1.316
1.307 1.400
1.412 1.413 1.427
TS endo s-cis
TS endo s-trans
1.400
1.361 1.397
1.391 1.365
1.388
2.600
1.942 2.504
1.411
2.003
1.418 1.429 1.400
1.247 1.239
1.311 1.309
TS exo s-cis
TS exo s-trans
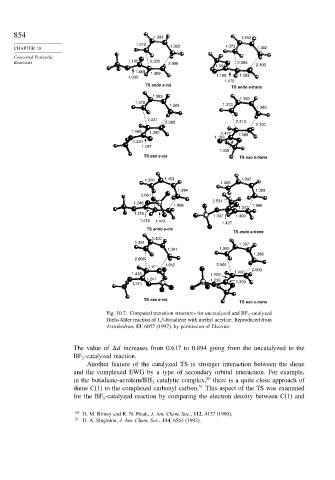
Fig. 10.7. Computed transition structures for uncatalyzed and BF 3 -catalyzed
Diels-Alder reaction of 1,3-butadiene with methyl acrylate. Reproduced from
Tetrahedron, 53, 6057 (1997), by permission of Elsevier.
The value of d increases from 0.617 to 0.894 going from the uncatalyzed to the
BF -catalyzed reaction.
3
Another feature of the catalyzed TS is stronger interaction between the diene
and the complexed EWG by a type of secondary orbital interaction. For example,
in the butadiene-acrolein/BH catalytic complex, 50 there is a quite close approach of
3
diene C(1) to the complexed carbonyl carbon. 51 This aspect of the TS was examined
for the BF -catalyzed reaction by comparing the electron density between C(1) and
3
50 D. M. Birney and K. N. Houk, J. Am. Chem. Soc., 112, 4127 (1990).
51
D. A. Singleton, J. Am. Chem. Soc., 114, 6563 (1992).