Page 871 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 871
1.428 3 855
4
5 1.364 1.380
SECTION 10.2
2
The Diels-Alder Reaction
2.827
2.209 2.805 1.627
6 1.269
1
1.380 1.422
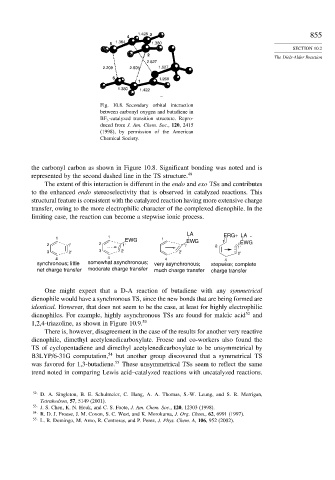
Fig. 10.8. Secondary orbital interaction
between carbonyl oxygen and butadiene in
BF 3 -catalyzed transition structure. Repro-
duced from J. Am. Chem. Soc., 120, 2415
(1998), by permission of the American
Chemical Society.
the carbonyl carbon as shown in Figure 10.8. Significant bonding was noted and is
represented by the second dashed line in the TS structure. 49
The extent of this interaction is different in the endo and exo TSs and contributes
to the enhanced endo stereoselectivity that is observed in catalyzed reactions. This
structural feature is consistent with the catalyzed reaction having more extensive charge
transfer, owing to the more electrophilic character of the complexed dienophile. In the
limiting case, the reaction can become a stepwise ionic process.
LA ERG+ LA -
1 1 EWG 1 EWG
2 1’ 2 1' 1’ 2 1 1’ EWG
3 2’ 3 2' 2’ 2’
4 4 4 4
synchronous; little somewhat asynchronous; very asynchronous; stepwise; complete
net charge transfer moderate charge transfer much charge transfer charge transfer
One might expect that a D-A reaction of butadiene with any symmetrical
dienophile would have a synchronous TS, since the new bonds that are being formed are
identical. However, that does not seem to be the case, at least for highly electrophilic
dienophiles. For example, highly asynchronous TSs are found for maleic acid 52 and
1,2,4-triazoline, as shown in Figure 10.9. 53
There is, however, disagreement in the case of the results for another very reactive
dienophile, dimethyl acetylenedicarboxylate. Froese and co-workers also found the
TS of cyclopentadiene and dimethyl acetylenedicarboxylate to be unsymmetrical by
B3LYP/6-31G computation, 54 but another group discovered that a symmetrical TS
was favored for 1,3-butadiene. 55 These unsymmetrical TSs seem to reflect the same
trend noted in comparing Lewis acid–catalyzed reactions with uncatalyzed reactions.
52
D. A. Singleton, B. E. Schulmeier, C. Hang, A. A. Thomas, S.-W. Leung, and S. R. Merrigan,
Tetrahedron, 57, 5149 (2001).
53 J. S. Chen, K. N. Houk, and C. S. Foote, J. Am. Chem. Soc., 120, 12303 (1998).
54 R. D. J. Froese, J. M. Coxon, S. C. West, and K. Morokuma, J. Org. Chem., 62, 6991 (1997).
55
L. R. Domingo, M. Arno, R. Contreras, and P. Perez, J. Phys. Chem. A, 106, 952 (2002).