Page 874 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 874
858
2.87
CHAPTER 10 H3
(2.90)
Concerted Pericyclic
Reactions
H4out
H4in
3.05
2.11 3.00 (2.98)
(2.17) (2.98)
2.51
(2.64)
2.94
2.09 2.94 (2.96)
(2.14) (2.93)
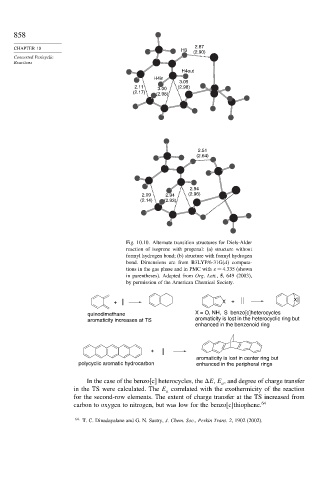
Fig. 10.10. Alternate transition structures for Diels-Alder
reaction of isoprene with propenal: (a) structure without
formyl hydrogen bond; (b) structure with formyl hydrogen
bond. Dimensions are from B3LYP/6-31G(d) computa-
tions in the gas phase and in PMC with = 4 335 (shown
in parentheses). Adapted from Org. Lett., 5, 649 (2003),
by permission of the American Chemical Society.
+ X + X
quinodimethane X = O, NH, S benzo[c]heterocycles
aromaticity increases at TS aromaticity is lost in the heterocyclic ring but
enhanced in the benzenoid ring
+
aromaticity is lost in center ring but
polycyclic aromatic hydrocarbon enhanced in the peripheral rings
In the case of the benzo[c] heterocycles, the E, E , and degree of charge transfer
a
in the TS were calculated. The E correlated with the exothermicity of the reaction
a
for the second-row elements. The extent of charge transfer at the TS increased from
carbon to oxygen to nitrogen, but was low for the benzo[c]thiophene. 64
64
T. C. Dinadayalane and G. N. Sastry, J. Chem. Soc., Perkin Trans. 2, 1902 (2002).