Page 875 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 875
859
NH O S SECTION 10.2
The Diels-Alder Reaction
E a 10.8 21.4 15.4 24.7
ΔE – 48.8 –16.2 –29.8 –27.1
c.t. 0.022 0.092 0.043 0.001
These results are consistent with experimental results. Polycyclic aromatic hydro-
carbons are moderately reactive as the diene component of Diels-Alder reactions.
Although benzene and naphthalene show no reactivity toward maleic anhydride at
65
90 C, anthracene does give an adduct. A variety of dienophiles react with anthracene,
including benzoquinone, dimethyl fumarate, nitroethene, and phenyl vinyl sulfoxide. 66
The addition occurs at the center ring. There is no net loss of resonance stabilization,
since the anthracene ring (resonance energy = 1 60eV) is replaced by two benzenoid
rings (total resonance energy = 2×0 87 = 1 74eV). 67
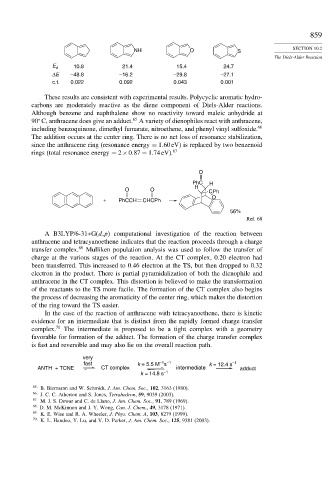
O
PhC H
H
O O CPh
O
+ PhCCH CHCPh
56%
Ref. 68
A B3LYP/6-31+G(d,p) computational investigation of the reaction between
anthracene and tetracyanoethene indicates that the reaction proceeds through a charge
transfer complex. 69 Mulliken population analysis was used to follow the transfer of
charge at the various stages of the reaction. At the CT complex, 0.20 electron had
been transferred. This increased to 0.46 electron at the TS, but then dropped to 0.32
electron in the product. There is partial pyramidalization of both the dienophile and
anthracene in the CT complex. This distortion is believed to make the transformation
of the reactants to the TS more facile. The formation of the CT complex also begins
the process of decreasing the aromaticity of the center ring, which makes the distortion
of the ring toward the TS easier.
In the case of the reaction of anthracene with tetracyanoethene, there is kinetic
evidence for an intermediate that is distinct from the rapidly formed charge transfer
complex. 70 The intermediate is proposed to be a tight complex with a geometry
favorable for formation of the adduct. The formation of the charge transfer complex
is fast and reversible and may also lie on the overall reaction path.
very
–1 –1
fast k = 5.5 M s k = 12.4 s –1
ANTH + TCNE CT complex intermediate adduct
k = 14.8 s –1
65 B. Biermann and W. Schmidt, J. Am. Chem. Soc., 102, 3163 (1980).
66 J. C. C. Atherton and S. Jones, Tetrahedron, 59, 9039 (2003).
67
M. J. S. Dewar and C. de Llano, J. Am. Chem. Soc., 91, 789 (1969).
68 D. M. McKinnon and J. Y. Wong, Can. J. Chem., 49, 3178 (1971).
69 K. E. Wise and R. A. Wheeler, J. Phys. Chem. A, 103, 8279 (1999).
70
K. L. Handoo, Y. Lu, and V. D. Parker, J. Am. Chem. Soc., 125, 9381 (2003).