Page 876 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 876
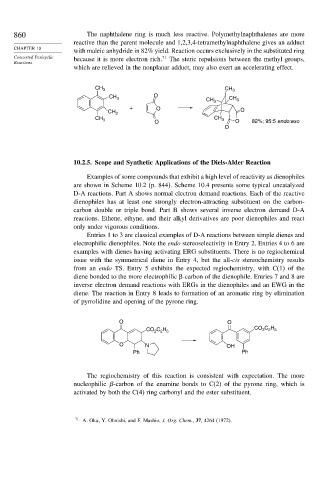
860 The naphthalene ring is much less reactive. Polymethylnaphthalenes are more
reactive than the parent molecule and 1,2,3,4-tetramethylnaphthalene gives an adduct
CHAPTER 10
with maleic anhydride in 82% yield. Reaction occurs exclusively in the substituted ring
Concerted Pericyclic because it is more electron rich. 71 The steric repulsions between the methyl groups,
Reactions
which are relieved in the nonplanar adduct, may also exert an accelerating effect.
CH 3 CH 3
CH 3 O CH 3 CH 3
+ O
CH 3 O
CH CH 3
3
O O 82%; 95:5 endo:exo
O
10.2.5. Scope and Synthetic Applications of the Diels-Alder Reaction
Examples of some compounds that exhibit a high level of reactivity as dienophiles
are shown in Scheme 10.2 (p. 844). Scheme 10.4 presents some typical uncatalyzed
D-A reactions. Part A shows normal electron demand reactions. Each of the reactive
dienophiles has at least one strongly electron-attracting substituent on the carbon-
carbon double or triple bond. Part B shows several inverse electron demand D-A
reactions. Ethene, ethyne, and their alkyl derivatives are poor dienophiles and react
only under vigorous conditions.
Entries 1 to 3 are classical examples of D-A reactions between simple dienes and
electrophilic dienophiles. Note the endo stereoselectivity in Entry 2. Entries 4 to 6 are
examples with dienes having activating ERG substituents. There is no regiochemical
issue with the symmetrical diene in Entry 4, but the all-cis stereochemistry results
from an endo TS. Entry 5 exhibits the expected regiochemistry, with C(1) of the
diene bonded to the more electrophilic -carbon of the dienophile. Entries 7 and 8 are
inverse electron demand reactions with ERGs in the dienophiles and an EWG in the
diene. The reaction in Entry 8 leads to formation of an aromatic ring by elimination
of pyrrolidine and opening of the pyrone ring.
O O
CO C H CO C H
2 2 5
2 2 5
O N OH
Ph Ph
The regiochemistry of this reaction is consistent with expectation. The more
nucleophilic -carbon of the enamine bonds to C(2) of the pyrone ring, which is
activated by both the C(4) ring carbonyl and the ester substituent.
71
A. Oku, Y. Ohnishi, and F. Mashio, J. Org. Chem., 37, 4264 (1972).