Page 877 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 877
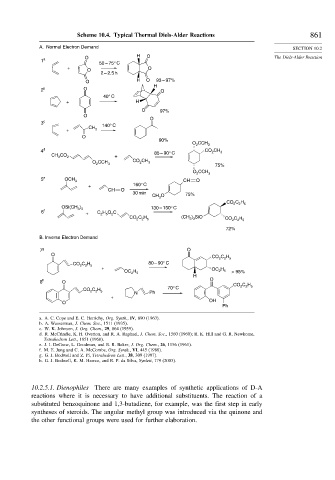
Scheme 10.4. Typical Thermal Diels-Alder Reactions 861
A. Normal Electron Demand SECTION 10.2
H O
O The Diels-Alder Reaction
1 a
50 – 75° C
+ O
O
2 – 2.5 h
H O 93 – 97%
O
H
2 b O O
40° C
+ H
O 97%
O
O
3 c 140° C
CH
+ 3
O
90%
O CCH 3
2
4 d 85 – 90° C CO CH 3
2
CH CO 2 +
3
O CCH CO CH 3
2
2 3
75%
O CCH 3
2
5 e OCH CH O
3
+ 160° C
CH O
30 min 75%
CH O
3
CO C H
2 2 5
)
OSi(CH 3 3 130 – 150° C
6 f C H O C
+ 2 5 2
CO C H (CH ) SiO CO C H
2 2 5 3 3 2 2 5
72%
B. Inverse Electron Demand
7 g O
O
CO C H
2 2 5
C H 80 – 90° C
CO 2 2 5
+ OC 2 H
OC 2 H 5 5 > 95%
H
8 h O O
CO C H
70° C 2 2 5
CO C H
2
5
2
N Ph
+
O OH
Ph
a. A. C. Cope and E. C. Herrichy, Org. Synth., IV, 890 (1963).
b. A. Wasserman, J. Chem. Soc., 1511 (1935).
c. W. K. Johnson, J. Org. Chem., 29, 864 (1959).
d. R. McCrindle, K. H. Overton, and R. A. Raphael, J. Chem. Soc., 1560 (1960); R. K. Hill and G. R. Newkome,
Tetrahedron Lett., 1851 (1968).
e. J. I. DeGraw, L. Goodman, and B. R. Baker, J. Org. Chem., 26, 1156 (1961).
f. M. E. Jung and C. A. McCombs, Org. Synth., VI, 445 (1988).
g. G. J. Bodwell and Z. Pi, Tetrahedron Lett., 38, 309 (1997).
h. G. J. Bodwell, K. M. Hawco, and R. P. da Silva, Synlett, 179 (2003).
10.2.5.1. Dienophiles There are many examples of synthetic applications of D-A
reactions where it is necessary to have additional substituents. The reaction of a
substituted benzoquinone and 1,3-butadiene, for example, was the first step in early
syntheses of steroids. The angular methyl group was introduced via the quinone and
the other functional groups were used for further elaboration.