Page 878 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 878
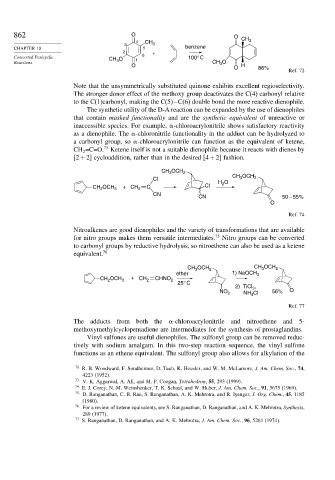
862 O O
4 CH 3
3 CH 3
CHAPTER 10 5 benzene
2 +
Concerted Pericyclic CH O 6 100° C
Reactions 3 1 CH O
3
O O H
86%
Ref. 72
Note that the unsymmetrically substituted quinone exhibits excellent regioselectivity.
The stronger donor effect of the methoxy group deactivates the C(4) carbonyl relative
to the C(1)carbonyl, making the C(5)−C(6) double bond the more reactive dienophile.
The synthetic utility of the D-A reaction can be expanded by the use of dienophiles
that contain masked functionality and are the synthetic equivalent of unreactive or
inaccessible species. For example, -chloroacrylonitrile shows satisfactory reactivity
as a dienophile. The -chloronitrile functionality in the adduct can be hydrolyzed to
a carbonyl group, so -chloroacrylonitrile can function as the equivalent of ketene,
73
CH =C=O. Ketene itself is not a suitable dienophile because it reacts with dienes by
2
[2+2] cycloaddition, rather than in the desired [4+2] fashion.
CH 3 OCH 2
CH OCH
Cl H O 3 2
CH OCH 3 + CH 2 C Cl 2
2
CN
CN 50 – 55%
O
Ref. 74
Nitroalkenes are good dienophiles and the variety of transformations that are available
for nitro groups makes them versatile intermediates. 75 Nitro groups can be converted
to carbonyl groups by reductive hydrolysis, so nitroethene can also be used as a ketene
equivalent. 76
CH OCH 2 CH OCH 2
3
3
ether 1) NaOCH 3
OCH + CH CHNO
CH 2 3 2 2
25° C
2) TiCl ,
3
NO 2 NH Cl 56% O
4
Ref. 77
The adducts from both the -chloroacrylonitrile and nitroethene and 5-
methoxymethylcyclopentadiene are intermediates for the synthesis of prostaglandins.
Vinyl sulfones are useful dienophiles. The sulfonyl group can be removed reduc-
tively with sodium amalgam. In this two-step reaction sequence, the vinyl sulfone
functions as an ethene equivalent. The sulfonyl group also allows for alkylation of the
72
R. B. Woodward, F. Sondheimer, D. Taub, K. Heusler, and W. M. McLamore, J. Am. Chem. Soc., 74,
4223 (1952).
73 V. K. Aggarwal, A. Ali, and M. P. Coogan, Tetrahedron, 55, 293 (1999).
74
E. J. Corey, N. M. Weinshenker, T. K. Schaaf, and W. Huber, J. Am. Chem. Soc., 91, 5675 (1969).
75
D. Ranganathan, C. B. Rao, S. Ranganathan, A. K. Mehrotra, and R. Iyengar, J. Org. Chem., 45, 1185
(1980).
76 For a review of ketene equivalents, see S. Ranganathan, D. Ranganathan, and A. K. Mehrotra, Synthesis,
289 (1977).
77
S. Ranganathan, D. Ranganathan, and A. K. Mehrotra, J. Am. Chem. Soc., 96, 5261 (1974).