Page 879 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 879
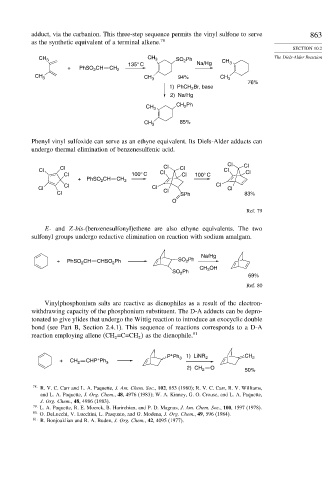
adduct, via the carbanion. This three-step sequence permits the vinyl sulfone to serve 863
as the synthetic equivalent of a terminal alkene. 78
SECTION 10.2
CH 3 CH 3 SO Ph The Diels-Alder Reaction
2
135° C Na/Hg CH 3
+ PhSO CH CH 2
2
CH 3 CH 3 94% CH 3
76%
1) PhCH 2 Br, base
2) Na/Hg
CH 3 CH Ph
2
85%
CH 3
Phenyl vinyl sulfoxide can serve as an ethyne equivalent. Its Diels-Alder adducts can
undergo thermal elimination of benzenesulfenic acid.
Cl Cl
Cl
Cl Cl Cl Cl Cl Cl
Cl 100° C Cl 100° C
+ PhSO CH CH 2
2
Cl Cl
Cl Cl Cl
Cl Cl SPh 83%
O
Ref. 79
E- and Z-bis-(benzenesulfonyl)ethene are also ethyne equivalents. The two
sulfonyl groups undergo reductive elimination on reaction with sodium amalgam.
Na/Hg
+ PhSO CH CHSO Ph SO Ph
2
2
2
CH 3 OH
Ph
SO 2
69%
Ref. 80
Vinylphosphonium salts are reactive as dienophiles as a result of the electron-
withdrawing capacity of the phosphonium substituent. The D-A adducts can be depro-
tonated to give ylides that undergo the Wittig reaction to introduce an exocyclic double
bond (see Part B, Section 2.4.1). This sequence of reactions corresponds to a D-A
reaction employing allene (CH =C=CH as the dienophile. 81
2
2
+
P Ph 3 1) LiNR 2 CH 2
+
+ CH 2 CHP Ph 3
2) CH 2 O 50%
78 R. V. C. Carr and L. A. Paquette, J. Am. Chem. Soc., 102, 853 (1980); R. V. C. Carr, R. V. Williams,
and L. A. Paquette, J. Org. Chem., 48, 4976 (1983); W. A. Kinney, G. O. Crouse, and L. A. Paquette,
J. Org. Chem., 48, 4986 (1983).
79
L. A. Paquette, R. E. Moerck, B. Harirchian, and P. D. Magnus, J. Am. Chem. Soc., 100, 1597 (1978).
80 O. DeLucchi, V. Lucchini, L. Pasquato, and G. Modena, J. Org. Chem., 49, 596 (1984).
81
R. Bonjouklian and R. A. Buden, J. Org. Chem., 42, 4095 (1977).