Page 881 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 881
10.2.6. Enantioselective Diels-Alder Reactions 865
10.2.6.1. Chiral Auxiliaries for Diels-Alder Reactions The highly ordered cyclic TS SECTION 10.2
of the Diels-Alder reaction permits design of reactants and catalysts that lead to The Diels-Alder Reaction
a preference between diastereomeric or enantiomeric adducts. (See Section 2.4) to
review the principles of diastereoselectivity and enantioselectivity.) One way to achieve
diastereoselectivity is to install a chiral auxiliary. 88 The cycloaddition proceeds to
give two diastereomeric products that can be separated and purified. Because of the
lower temperature and the greater stereoselectivity, the best diastereoselectivity is
often observed in Lewis acid–catalyzed reactions. Chiral esters and amides of acrylic
acid are particularly useful because the chiral auxiliary can be easily recovered by
hydrolysis of the purified adduct to give the enantiomerically pure carboxylic acid.
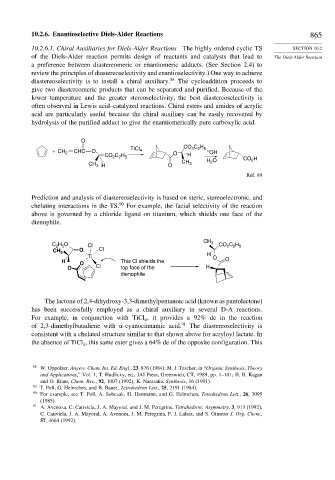
O
2 2 5
TiCl CO C H
+ CH 2 CHC O 4 O – OH
C H
CO 2 2 5 H CO H
CH H O 2
2
CH 3 H O 3
Ref. 89
Prediction and analysis of diastereoselectivity is based on steric, stereoelectronic, and
chelating interactions in the TS. 90 For example, the facial selectivity of the reaction
above is governed by a chloride ligand on titanium, which shields one face of the
dienophile.
CH
H O 3 C H
C 2 5 Cl CO 2 2 5
CH 3 O Cl
Ti H O
H O This Cl shields the O
O Cl top face of the H
dienophile
The lactone of 2,4-dihydroxy-3,3-dimethylpentanoic acid (known as pantolactone)
has been successfully employed as a chiral auxiliary in several D-A reactions.
For example, in conjunction with TiCl , it provides a 92% de in the reaction
4
of 2,3-dimethylbutadiene with -cyanocinnamic acid. 91 The diastereoselectivity is
consistent with a chelated structure similar to that shown above for acryloyl lactate. In
the absence of TiCl , this same ester gives a 64% de of the opposite configuration. This
4
88 W. Oppolzer, Angew. Chem. Int. Ed. Engl., 23, 876 (1984); M. J. Tascher, in “Organic Synthesis, Theory
and Applications,” Vol. 1, T. Hudlicky, ed., JAI Press, Greenwich, CT, 1989, pp. 1–101; H. B. Kagan
and O. Riant, Chem. Rev., 92, 1007 (1992); K. Narasaka, Synthesis, 16 (1991).
89
T. Poll, G. Helmchen, and B. Bauer, Tetrahedron Lett., 25, 2191 (1984).
90 For example, see T. Poll, A. Sobczak, H. Hartmann, and G. Helmchen, Tetrahedron Lett., 26, 3095
(1985).
91
A. Avenoza, C. Cativiela, J. A. Mayoral, and J. M. Peregrina, Tetrahedron: Asymmetry, 3, 913 (1992);
C. Cativiela, J. A. Mayoral, A. Avenoza, J. M. Peregrina, F. J. Lahoz, and S. Gimeno J. Org. Chem.,
57, 4664 (1992).