Page 882 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 882
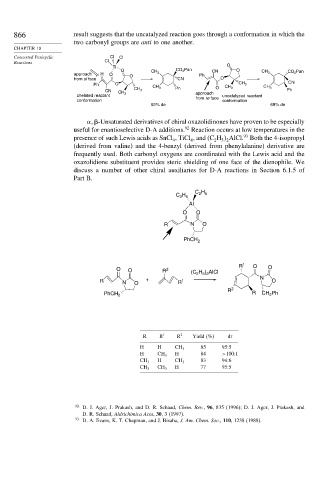
866 result suggests that the uncatalyzed reaction goes through a conformation in which the
two carbonyl groups are anti to one another.
CHAPTER 10
Concerted Pericyclic Cl Cl
Reactions Cl
Ti O
O CO 2 Pan CN O CO 2 Pan
approach H O O CH 3 Ph CH 3
from si face CN O
Ph O CH 3 CN
CH 3 Ph O CH 3 CH 3
CN CH 3 Ph
CH 3 approach
chelated reactant uncatalyzed reactant
conformation from re face conformation
92% de 69% de
-Unsaturated derivatives of chiral oxazolidinones have proven to be especially
92
useful for enantioselective D-A additions. Reaction occurs at low temperatures in the
93
presence of such Lewis acids as SnCl , TiCl , and (C H AlCl. Both the 4-isopropyl
4
4
2
5 2
(derived from valine) and the 4-benzyl (derived from phenylalanine) derivative are
frequently used. Both carbonyl oxygens are coordinated with the Lewis acid and the
oxazolidione substituent provides steric shielding of one face of the dienophile. We
discuss a number of other chiral auxiliaries for D-A reactions in Section 6.1.5 of
Part B.
C H
C H 2 5
2 5
Al
O O
R N O
PhCH 2
R 1 O
O O R 2 (C H ) AlCl O
2 5 2
R N O + R 1 N O
R 2
2
PhCH 2 R CH Ph
R R 1 R 2 Yield (%) dr
H H CH 3 85 95:5
H CH 3 H 84 >100:1
H 83 94:6
CH 3 CH 3
H 77 95:5
CH 3 CH 3
92 D. J. Ager, J. Prakash, and D. R. Schaad, Chem. Rev., 96, 835 (1996); D. J. Ager, J. Prakash, and
D. R. Schaad, Aldrichimica Acta, 30, 3 (1997).
93
D. A. Evans, K. T. Chapman, and J. Bisaha, J. Am. Chem. Soc., 110, 1238 (1988).