Page 884 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 884
868
CHAPTER 10
Concerted Pericyclic C
Reactions H
N
α-Si face
Cu
O N
β
N
α
α-Re face
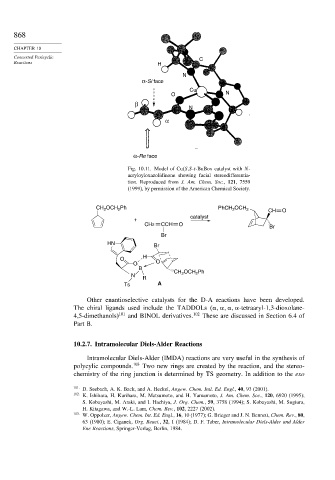
Fig. 10.11. Model of Cu(S,S-t-BuBox catalyst with N-
acryloyloxazolidinone showing facial stereodifferentia-
tion. Reproduced from J. Am. Chem. Soc., 121, 7559
(1999), by permission of the American Chemical Society.
CH OCH Ph PhCH OCH 2 CH O
2
2
2
catalyst
+
CH2 CCH O
Br
Br
HN
Br
O H
O O
B
CH OCH Ph
N 2 2
R
Ts A
Other enantioselective catalysts for the D-A reactions have been developed.
The chiral ligands used include the TADDOLs ( -tetraaryl-1,3-dioxolane-
4,5-dimethanols) 101 and BINOL derivatives. 102 These are discussed in Section 6.4 of
Part B.
10.2.7. Intramolecular Diels-Alder Reactions
Intramolecular Diels-Alder (IMDA) reactions are very useful in the synthesis of
polycylic compounds. 103 Two new rings are created by the reaction, and the stereo-
chemistry of the ring junction is determined by TS geometry. In addition to the exo
101
D. Seebach, A. K. Back, and A. Heckel, Angew. Chem. Intl. Ed. Engl., 40, 93 (2001).
102 K. Ishihara, H. Kurihara, M. Matsumoto, and H. Yamamoto, J. Am. Chem. Soc., 120, 6920 (1995);
S. Kobayashi, M. Araki, and I. Hachiya, J. Org. Chem., 59, 3758 (1994); S. Kobayashi, M. Sugiura,
H. Kitagawa, and W.-L. Lam, Chem. Rev., 102, 2227 (2002).
103
W. Oppolzer, Angew. Chem. Int. Ed. Engl., 16, 10 (1977); G. Brieger and J. N. Bennett, Chem. Rev., 80,
63 (1980); E. Ciganek, Org. React., 32, 1 (1984); D. F. Taber, Intramolecular Diels-Alder and Alder
Ene Reactions, Springer-Verlag, Berlin, 1984.