Page 956 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 956
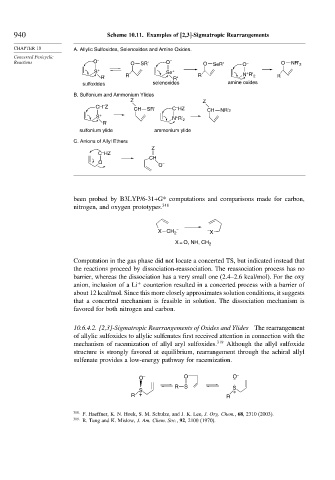
940 Scheme 10.11. Examples of [2,3]-Sigmatropic Rearrangements
CHAPTER 10 A. Allylic Sulfoxides, Selenoxides and Amine Oxides.
Concerted Pericyclic
Reactions O – O SR' O – O SeR' O – O NR' 2
S + Se + +
R' R R' R N R' 2 R
sulfoxides selenoxides amine oxides
B. Sulfonium and Ammonium Ylides
Z Z
–
CH Z –
CH SR' C HZ CH NR'2
S + +
N R' 2
R'
sulfonium ylide ammonium ylide
C. Anions of Allyl Ethers
Z
–
C HZ
CH
O –
O
been probed by B3LYP/6-31+G* computations and comparisons made for carbon,
nitrogen, and oxygen prototypes. 318
XCH 2 – – X
X = O, NH, CH 2
Computation in the gas phase did not locate a concerted TS, but indicated instead that
the reactions proceed by dissociation-reassociation. The reassociation process has no
barrier, whereas the dissociation has a very small one (2.4–2.6 kcal/mol). For the oxy
+
anion, inclusion of a Li counterion resulted in a concerted process with a barrier of
about 12 kcal/mol. Since this more closely approximates solution conditions, it suggests
that a concerted mechanism is feasible in solution. The dissociation mechanism is
favored for both nitrogen and carbon.
10.6.4.2. [2,3]-Sigmatropic Rearrangements of Oxides and Ylides The rearrangement
of allylic sulfoxides to allylic sulfenates first received attention in connection with the
mechanism of racemization of allyl aryl sulfoxides. 319 Although the allyl sulfoxide
structure is strongly favored at equilibrium, rearrangement through the achiral allyl
sulfenate provides a low-energy pathway for racemization.
O – O O –
R S S
S +
R + R
318 F. Haeffner, K. N. Houk, S. M. Schulze, and J. K. Lee, J. Org. Chem., 68, 2310 (2003).
319
R. Tang and K. Mislow, J. Am. Chem. Soc., 92, 2100 (1970).