Page 957 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 957
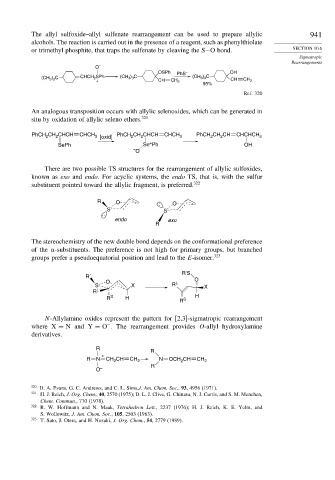
The allyl sulfoxide–allyl sulfenate rearrangement can be used to prepare allylic 941
alcohols. The reaction is carried out in the presence of a reagent, such as phenylthiolate
or trimethyl phosphite, that traps the sulfenate by cleaving the S−O bond. SECTION 10.6
Sigmatropic
Rearrangements
O –
OSPh PhS – OH
(CH 3 ) 3 C CHCH 2 SPh (CH 3 ) 3 C (CH 3 ) 3 C CH
+ CH CH 2 CH 2
95%
Ref. 320
An analogous transposition occurs with allylic selenoxides, which can be generated in
situ by oxidation of allylic seleno ethers. 321
PhCH 2 CH 2 CHCH CHCH 3 [oxid] PhCH 2 CH 2 CHCH CHCH 3 PhCH 2 CH 2 CH CHCHCH 3
+
SePh Se Ph OH
–
O
There are two possible TS structures for the rearrangement of allylic sulfoxides,
known as exo and endo. For acyclic systems, the endo TS, that is, with the sulfur
substituent pointed toward the allylic fragment, is preferred. 322
R O O
S : S
:
endo exo
R
The stereochemistry of the new double bond depends on the conformational preference
of the -substituents. The preference is not high for primary groups, but branched
groups prefer a pseudoequatorial position and lead to the E-isomer. 323
R′ R'S
O L O
S X R X
R L
R S H S H
R
N-Allylamine oxides represent the pattern for [2,3]-sigmatropic rearrangement
–
where X = N and Y = O . The rearrangement provides O-allyl hydroxylamine
derivatives.
R
R
+
R N CH CH CH 2 N OCH CH CH 2
2
2
R
O –
320 D. A. Evans, G. C. Andrews, and C. L. Sims,J. Am. Chem. Soc., 93, 4956 (1971).
321
H. J. Reich, J. Org. Chem., 40, 2570 (1975); D. L. J. Clive, G. Chittatu, N. J. Curtis, and S. M. Menchen,
Chem. Commun., 770 (1978).
322 R. W. Hoffmann and N. Maak, Tetrahedron Lett., 2237 (1976); H. J. Reich, K. E. Yelm, and
S. Wollowitz, J. Am. Chem. Soc., 105, 2503 (1983).
323
T. Sato, J. Otera, and H. Nozaki, J. Org. Chem., 54, 2779 (1989).