Page 961 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 961
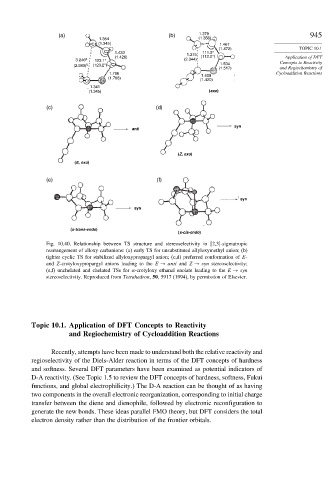
(a) (b) 1.379 945
1.354 (1.356)
C1 (1.345) 1.467
(1.472) TOPIC 10.1
C2 1.433 111.5°
(1.426) 1.275 (112.0°) Application of DFT
3.246 a 123.1° (2.344)
(2.983) b (123.2°) C3 1.534 Concepts to Reactivity
(1.517) and Regiochemistry of
1.766 1.409 Cycloaddition Reactions
(1.768)
C5 C4 (1.420)
1.341
(1.345) (exo)
(c) (d)
syn
anti
(Z, exo)
(E, exo)
(e) (f)
syn
syn
(s-trans-endo)
(s-cis-endo)
Fig. 10.40. Relationship between TS structure and stereoselectivity in [2,3]-sigmatropic
rearrangement of alloxy carbanions: (a) early TS for unsubstituted allyloxymethyl anion; (b)
tighter cyclic TS for stabilized allyloxypropargyl anion; (c,d) preferred conformation of E-
and Z-crotyloxypropargyl anions leading to the E → anti and Z → syn stereoselectivity;
(e,f) unchelated and chelated TSs for -crotyloxy ethanal enolate leading to the E → syn
stereoselectivity. Reproduced from Tetrahedron, 50, 5917 (1994), by permission of Elsevier.
Topic 10.1. Application of DFT Concepts to Reactivity
and Regiochemistry of Cycloaddition Reactions
Recently, attempts have been made to understand both the relative reactivity and
regioselectivity of the Diels-Alder reaction in terms of the DFT concepts of hardness
and softness. Several DFT parameters have been examined as potential indicators of
D-A reactivity. (See Topic 1.5 to review the DFT concepts of hardness, softness, Fukui
functions, and global electrophilicity.) The D-A reaction can be thought of as having
two components in the overall electronic reorganization, corresponding to initial charge
transfer between the diene and dienophile, followed by electronic reconfiguration to
generate the new bonds. These ideas parallel FMO theory, but DFT considers the total
electron density rather than the distribution of the frontier orbitals.