Page 963 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 963
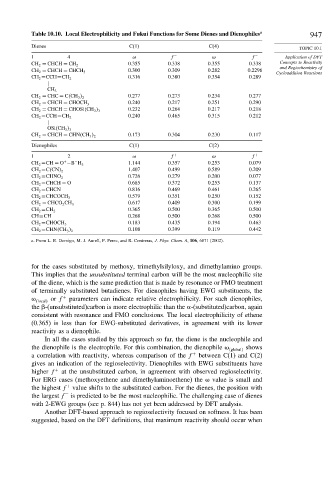
Table 10.10. Local Electrophilicity and Fukui Functions for Some Dienes and Dienophiles a 947
Dienes C(1) C(4)
TOPIC 10.1
1 4 f − f − Application of DFT
0.355 0.338 0.355 0.338 Concepts to Reactivity
CH 2 = CHCH = CH 2
and Regiochemistry of
0.300 0.309 0.282 0.2296
CH 2 = CHCH = CHCH 3
Cycloaddition Reactions
0.316 0.380 0.354 0.289
CH 2 =CCH=CH 2
CH 3
0.277 0.273 0.234 0.277
CH 2 = CHC = C CH 3 2
0.240 0.217 0.251 0.290
CH 2 = CHCH = CHOCH 3
0.232 0.264 0.217 0.218
CH 2 = CHCH = CHOSi CH 3 3
0.240 0.465 0.315 0.212
CH 2 =CCH=CH 2
OSi CH 3 3
0.173 0.304 0.230 0.117
CH 2 = CHCH = CHN CH 3 2
Dienophiles C(1) C(2)
1 2 f + f +
+ − 1.144 0.357 0.253 0.079
CH 2 =CH = O −B H 3
1.407 0.499 0.589 0.209
CH 2 =C CN 2
0.726 0.279 0.200 0.077
CH 2 =CHNO 2
CH 2 =CHCH = O 0.685 0.372 0.253 0.137
CH 2 =CHCN 0.816 0.469 0.461 0.265
0.579 0.351 0.250 0.152
CH 2 =CHCOCH 3
0.617 0.409 0.300 0.199
CH 2 = CHCO 2 CH 3
0.365 0.500 0.365 0.500
CH 2 =CH 2
CH≡CH 0.268 0.500 0.268 0.500
0.183 0.435 0.194 0.463
CH 2 =CHOCH 3
0.108 0.399 0.119 0.442
CH 2 =CHN CH 3 2
a. From L. R. Domigo, M. J. Aurell, P. Perez, and R. Contreras, J. Phys. Chem. A, 106, 6871 (2002).
for the cases substituted by methoxy, trimethylsilyloxy, and dimethylamino groups.
This implies that the unsubstituted terminal carbon will be the most nucleophilic site
of the diene, which is the same prediction that is made by resonance or FMO treatment
of terminally substituted butadienes. For dienophiles having EWG substituents, the
or f + parameters can indicate relative electrophilicity. For such dienophiles,
local
the -(unsubstituted)carbon is more electrophilic than the -(substituted)carbon, again
consistent with resonance and FMO conclusions. The local electrophilicity of ethene
(0.365) is less than for EWG-substituted derivatives, in agreement with its lower
reactivity as a dienophile.
In all the cases studied by this approach so far, the diene is the nucleophile and
the dienophile is the electrophile. For this combination, the dienophile global shows
a correlation with reactivity, whereas comparison of the f + between C(1) and C(2)
gives an indication of the regioselectivity. Dienophiles with EWG substituents have
higher f + at the unsubstituted carbon, in agreement with observed regioselectivity.
For ERG cases (methoxyethene and dimethylaminoethene) the value is small and
the highest f value shifts to the substituted carbon. For the dienes, the position with
+
–
the largest f is predicted to be the most nucleophilic. The challenging case of dienes
with 2-EWG groups (see p. 844) has not yet been addressed by DFT analysis.
Another DFT-based approach to regioselectivity focused on softness. It has been
suggested, based on the DFT definitions, that maximum reactivity should occur when