Page 966 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 966
950
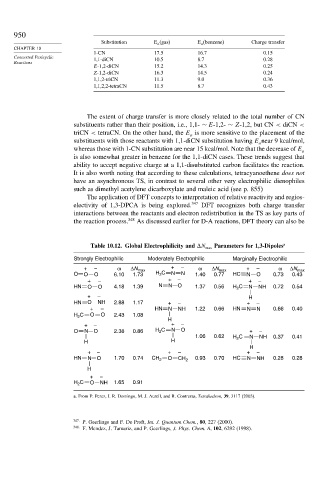
Substitution E a (gas) E a (benzene) Charge transfer
CHAPTER 10
1-CN 17.5 16.7 0.15
Concerted Pericyclic 1,1-diCN 10.5 8.7 0.28
Reactions
E-1,2-diCN 15.2 14.3 0.25
Z-1,2-diCN 16.3 14.5 0.24
1,1,2-triCN 11.3 9.0 0.36
1,1,2,2-tetraCN 11.5 8.7 0.43
The extent of charge transfer is more closely related to the total number of CN
substituents rather than their position, i.e., 1,1- ∼ E-1,2- ∼ Z-1,2, but CN < diCN <
triCN < tetraCN. On the other hand, the E is more sensitive to the placement of the
a
substituents with those reactants with 1,1-diCN substitution having E near 9 kcal/mol,
a
whereas those with 1-CN substitution are near 15 kcal/mol. Note that the decrease of E
a
is also somewhat greater in benzene for the 1,1-diCN cases. These trends suggest that
ability to accept negative charge at a 1,1-disubstituted carbon facilitates the reaction.
It is also worth noting that according to these calculations, tetracyanoethene does not
have an asynchronous TS, in contrast to several other very electrophilic dienophiles
such as dimethyl acetylene dicarboxylate and maleic acid (see p. 855)
The application of DFT concepts to interpretation of relative reactivity and regios-
electivity of 1,3-DPCA is being explored. 347 DFT recognizes both charge transfer
interactions between the reactants and electron redistribution in the TS as key parts of
the reaction process. 348 As discussed earlier for D-A reactions, DFT theory can also be
Table 10.12. Global Electrophilicity and N max Parameters for 1,3-Dipoles a
Strongly Electrophilic Moderately Electrophilic Marginally Electrophilic
+ – ω ΔN max + – ω ΔN max + – ω ΔN max
O O O 6.10 1.73 H 2 C N N 1.40 0.77 HC N O 0.73 0.43
+ – + – + –
HN O O 4.18 1.39 N N O 1.37 0.56 H C N NH 0.72 0.54
2
+ – H
HN O NH 2.88 1.17 + – + –
+ – HN N NH 1.22 0.66 HN N N 0.66 0.40
H C O O 2.43 1.08
2
H
+ – + –
O N O 2.38 0.86 H C N O + –
2
1.06 0.62 H C N NH 0.37 0.41
2
H H
H
+ – + – + –
HN N O 1.70 0.74 CH 2 O CH 2 0.93 0.70 HC N NH 0.28 0.28
H
+ –
H C O NH 1.65 0.91
2
a. From P. Perez, I. R. Domingo, M. J. Aurell, and R. Contreras, Tetrahedron, 39, 3117 (2003).
347 P. Geerlings and F. De Proft, Int. J. Quantum Chem., 80, 227 (2000).
348
F. Mendez, J. Tamariz, and P. Geerlings, J. Phys. Chem. A, 102, 6292 (1998).