Page 968 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 968
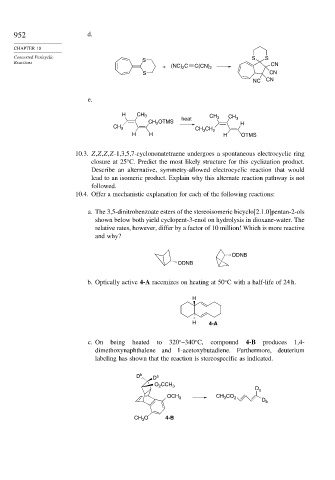
952 d.
CHAPTER 10
Concerted Pericyclic S S
Reactions S CN
+ (NC) C C(CN) 2
2
S CN
NC CN
e.
H CH 3 CH 3 CH 3
OTMS heat
CH 2 H
CH 3 CH CH 2
3
H H H OTMS
10.3. Z,Z,Z,Z-1,3,5,7-cyclononatetraene undergoes a spontaneous electrocyclic ring
closure at 25 C. Predict the most likely structure for this cyclization product.
Describe an alternative, symmetry-allowed electrocyclic reaction that would
lead to an isomeric product. Explain why this alternate reaction pathway is not
followed.
10.4. Offer a mechanistic explanation for each of the following reactions:
a. The 3,5-dinitrobenzoate esters of the stereoisomeric bicyclo[2.1.0]pentan-2-ols
shown below both yield cyclopent-3-enol on hydrolysis in dioxane-water. The
relative rates, however, differ by a factor of 10 million! Which is more reactive
and why?
ODNB
ODNB
b. Optically active 4-A racemizes on heating at 50 C with a half-life of 24 h.
H
H 4-A
c. On being heated to 320 –340 C, compound 4-B produces 1,4-
dimethoxynaphthalene and 1-acetoxybutadiene. Furthermore, deuterium
labeling has shown that the reaction is stereospecific as indicated.
D b D a
O CCH 3
2
D a
OCH 3 CH CO 2
3
D b
CH O 4-B
3